2011 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2010 – AUG 2011
Executive Summary
This report summarizes the 12-month period of research—September 1, 2010 through August 31, 2011—by the JPL-Titan team, hereinafter the NAI Titan team.
A series of coupled model simulations and novel laboratory experiments comprise the core research program of the NAI Titan team. The objective of this coordinated research is to understand the extent to which processes that could be active currently in Titan could lead to the formation of significant prebiotic molecular compounds, to be defined hereinafter as being composed of atoms of hydrogen, carbon, nitrogen, and oxygen. These processes might have been important in the early Earth environment and be on the path to the formation of life.
The NAI Titan research program is organized along the lines of three research themes—“Titan’s geology—places where organic chemistry can operate”, “The complexity of atmospheric organic chemistry”, and “The evolved chemical state of the Titan surface”.
An overview of what has occurred in each of the themes is presented in what follows.
Theme 1 – Titan’s geology—places where organic chemistry can operate:
The internal and external geologic evolution of Titan was investigated so as to constrain the environment in which organic evolution has proceeded over time. The evolution of the interior and leaching of salts from hydrated silicate interior to the ammonia-water ocean above was modeled. Clathrate density and stability was studied to determine the evolution of Titan crust and comparison was made with Cassini data. The model yields a plausible mechanism for the renewed or de novo onset of outgassing from the crust of Titan beginning ½-1 billion years ago, consistent with Cassini gravity and mass spectrometry data.
Reaction between hydrocarbons and water ice was modeled to assess the possible extent of prebiotic compound formation in this context. Interpretation of Cassini observations suggests that hydrocarbons remain in contact with the icy substrate for long period of time. The hydrocarbons should react with an icy crust and/or a methane-clathrate crust until clathration is complete.
A model of the Schumann resonance in Titan’s cavity formed by its ionosphere and a subsurface conductive layer has been improved and can be interpreted as a deep ammoniac-rich ocean. The major improvement was to take into account the variations of atmospheric conductivity with altitude. This improved model suggests that the ocean is 70 km (±10 km) deep.
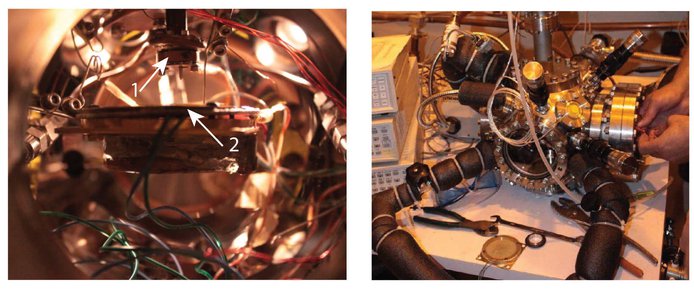
Laboratory work led to several results. Tholins are entrained in the subsurface during a methane rain. As the liquid evaporates, the tholins remain trapped in the subsurface. The JPL Titan chamber (Figure 1) also was used to test a rain drop sensor developed by a group of students at University of Idaho that could be embarked on future missions to Titan.
Theme 2 – The complexity of atmospheric organic chemistry:
The development of a master atmospheric model is nearing completion. Based on an older Titan model, the current model has been updated this year to include a treatment of chemical equilibrium, a description of aerosols, and a numerical model for condensation on and sublimation of atmospheric organic molecules from aerosol particles. To allow a global simulation of Titan atmospheric organic chemistry, the computer model is being recoded to support parallel processing.
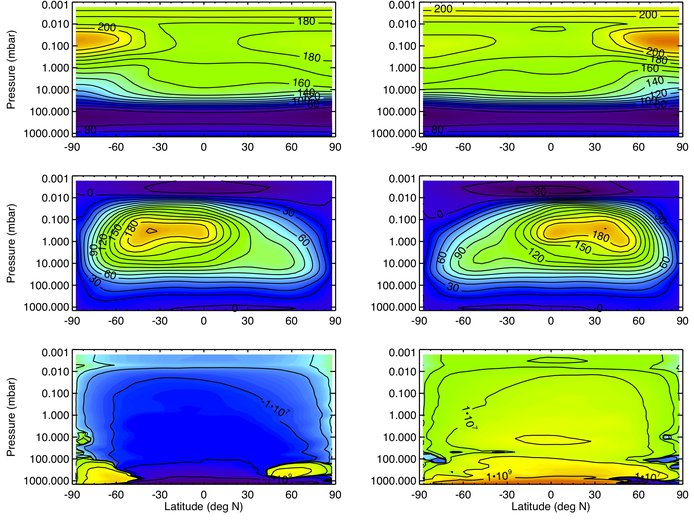
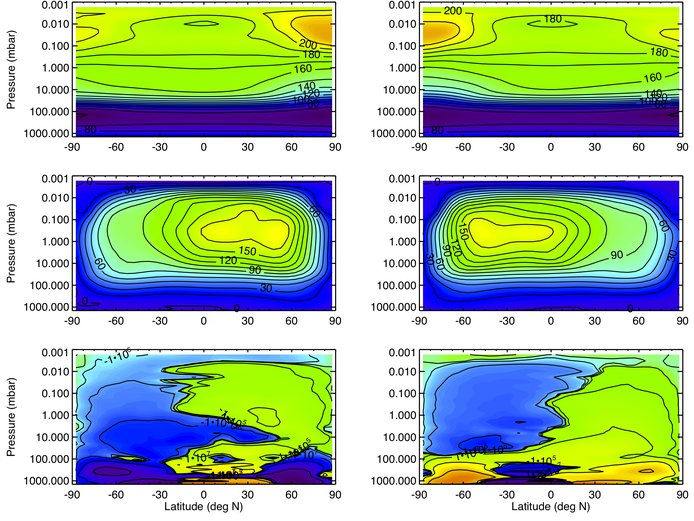
The chemical model requires a description of the background state of the atmosphere, specifically temperature and circulation as a function of latitude and longitude. To produce realistic Titan atmospheric profiles (winds, temperatures and densities) from the surface to ~1200km, for a variety of seasons and solar cycles, for use by other members of the NAI Titan project, the Titan General Circulation model (TGCM), simulating the atmosphere typically above 600 km, was revised to shift its lower boundary downward to eliminate the gap region from 600 km down to 430 km, the upper boundary of the lower/middle atmosphere Titan model (TitanWRF). There is now a “model coupling” framework to force the lower boundary of the TGCM using previously generated output from the upper portion of TitanWRF. In addition, the lower/middle atmosphere model now successfully reproduces the observed amount of stratospheric superrotation, a fundamental requirement in order for the model coupling to produce realistic results (see Figures 2 and 3).
A previously unsuspected seasonal change in the altitude of Titan’s detached haze layer was discovered and used to test current models of the formation of the haze and related dynamical and microphysical processes. The altitude of the detached haze in 84 Cassini ISS images from 2005 to 2011 was measured (Figure 4) and related to two current models of the haze formation process. A large-amplitude (150 km) drop in the altitude of the detached haze near equinox was identified. These observations provide a severe test of existing models, indeed, neither model is capable of explaining the totality of the measurements, although both models explain different features of the observations.
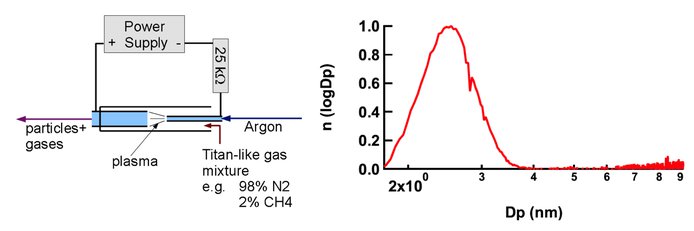
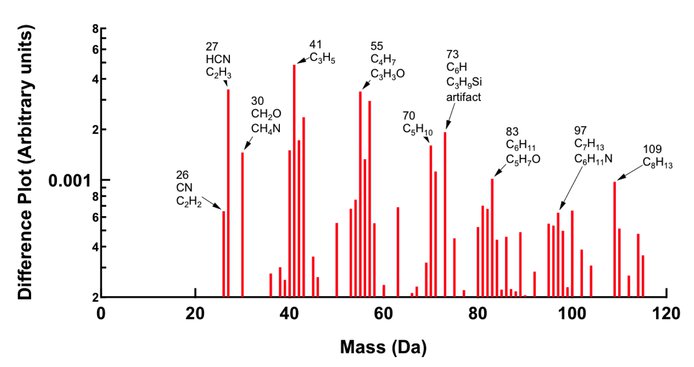
A quantitative understanding of the particle formation and growth in the Titan atmosphere is still unrealized. A microplasma reactor has been constructed to act as a source for tholin-like particles in our experiments. Illustrated in Figure 5a, the microplasma reactor employs an atmospheric pressure, hollow cathode discharge in a 180 μm diameter tube. The particles produced with this reactor are small, about 2-3 nm diameter, as shown in Figure 5b. The composition of the product particles, determined using an Aerodyne Research, Inc., Aerosol Mass Spectrometer (AMS), shown in Figure 6, is similar to that reported by Waite et al. (2005) for the ion-neutral mass spectrometer data obtained in a Titan flyby.
Only near-ultraviolet light at long wavelengths can penetrate to the deep Titan atmosphere, not being absorbed by atmospheric gas-phase species. Large hydrocarbons can absorb at these longer wavelengths. Condensed onto atmospheric particles, such hydrocarbons can undergo photochemical reactions initiated by absorption of near-ultraviolet photons. Dicyanoacetylene (C4N2) was synthesized and purified for spectroscopic and photochemical studies. Condensed ices of C4N2 showed production of nonvolatile polymeric material when irradiated at 355 nm, but not 532 nm (Figure 7). These studies unambiguously show that longer wavelength photochemistry of condensed Titan organics can occur at lower haze altitudes (~100 km) in Titan’s atmosphere. The optical properties are similar to those observed for Titan tholins.
Theme 3 – The evolved chemical state of the Titan surface:
A goal is to determine the potential role of mineral surfaces (i.e. meteorite fragments) in catalyzing reactions on Titan’s surface. There is also the possibility of low-energy electron and visible/UV photon stimulated chemistry on aggregates and organic aerosol surfaces. State-of-the art surface science and laser techniques are used to examine non-thermal reactions on low-temperature ice surfaces and mineral interfaces. The mineral samples are created by laser ablation and the reactions are examined using surface FTIR- spectroscopy, time-of-flight mass spectroscopy and temperature programmed desorption/reactions. Thus far the interaction of small organic molecules (HCN, C2H2, CH3NH2 and CH3CN) on mineral surfaces (silicates) and on organic and/or water ice substrates has been examined. Initial results indicate that reactive proton scattering occurs readily on organic/water ices at cryogenic temperature during low energy electron (5-50 eV) bombardment. This leads to the facile formation and ejection of complex polyatomic ions. Silicate minerals and nitrogen-rich tholins also likely play a significant role in the production of pre-biotic molecules since they can act as catalysts and reactive “substrates”. In the case of tholins, these complicated molecules can be the source of ionic species such as C2H3+, C2H5+, CH-, CN- and C2H- as well as reactive radicals such as CH, C2H, CN, etc.
Triboelectric reactions of complex organics and water ice are a potential chemical mechanism active in the dunes of Titan. Such reactions could possibly lead to dissociation of water molecules or incorporation of oxygen through free radical chemistry. In fact, recent work has demonstrated that electrical discharges occurring over water-ice in a simulated Titan environment do in fact lead to oxygen incorporation via the formation of nitromethane, esters, ethers, and aldehydes. During the saltation process, organic particles undergo charging due to friction between particles, and these processes have been measured to create electric fields up to 160 kV/m on Earth, well above the fields required to generate electrical discharges even at the higher pressures of Titan’s surface. An updated fluidized bed system has been constructed to simulate the agitation that particles may experience during saltation on Titan’s surface. As shown schematically in Figure 8a, a flow of nitrogen gas cooled by passing through a coil submerged in liquid N2 is employed to agitate a bed borosilicate glass particles 1 mm in diameter that have been coated with a thin film of an organic compound of interest. The device is shown in operation in Figure 8b. Once the system is cooled, a small amount of liquid water is added to the system to provide a water-ice source for oxygen incorporation. After a short period of agitation the beads become highly charged, and exhibit increased adhesion to each other and to the glass apparatus (Figure 8c). Initial experiments—using aminoacetonitrile, a possible precursor of biologically relevant compounds such as glycine—have shown no new product formation resulting from the putative triboelectric process.
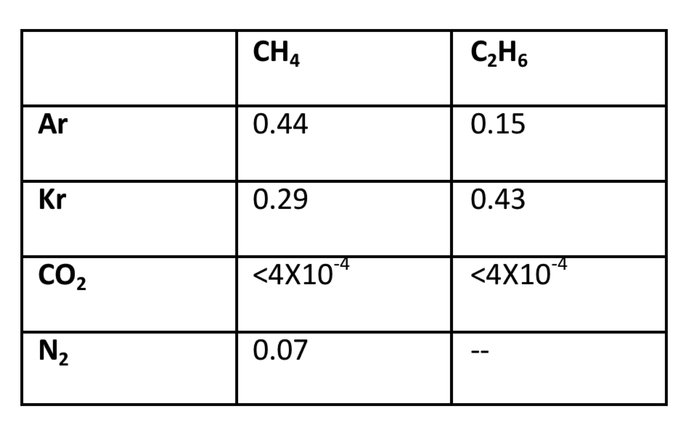
Measurements of the solubility of gases in liquid ethane and methane have been collected, using the quadrupole mass spectrometer system that was developed in the first year of this work. The solubilities of argon and krypton in liquid ethane and methane are listed in the table below (Table 1) and are consistent with theoretical values. The solubilities of several small aromatics are also currently being measured. This method of sampling and analysis of liquid hydrocarbon solutions could be easily adapted for in situ Titan missions with the goal of sampling Titan’s lakes.
Alternatively, atomistic simulations are being used to study the chemical environment of Titan’s hydrocarbon lakes. Given the dearth of polar molecules in these lakes, the solution-phase energetics and dynamics are governed by weak interactions that are usually overshadowed in aqueous environments. To gain insight into the nature of these interactions, enhanced sampling techniques are being used to study the pair-wise free energy of association, solvation free energies, and radial distribution functions of small non-polar solutes in the bulk solution of the hydrocarbon lakes. Surface properties of the hydrocarbons lakes are also of central interest. Simulations of methane at liquid-vapor phase coexistence and the Titan surface temperature are thus being performed. To explore the effect of solute and solvent polarizability in the non-polar lakes, molecular dynamics simulations that employ polarizable force fields (adiabatic shell model) are being used to model the aggregation and self-assembly of polarizable solutes in the hydrocarbon lakes. By tuning both the relative concentration of polarizable molecules and the degree of polarizability, the conditions that allow for self-assembly can be studied. The conditions necessary for non-polar aggregation in the simulations are being compared to the conditions present in the hydrocarbon lakes to draw conclusions as to the existence of such aggregates in the hydrocarbon lakes.
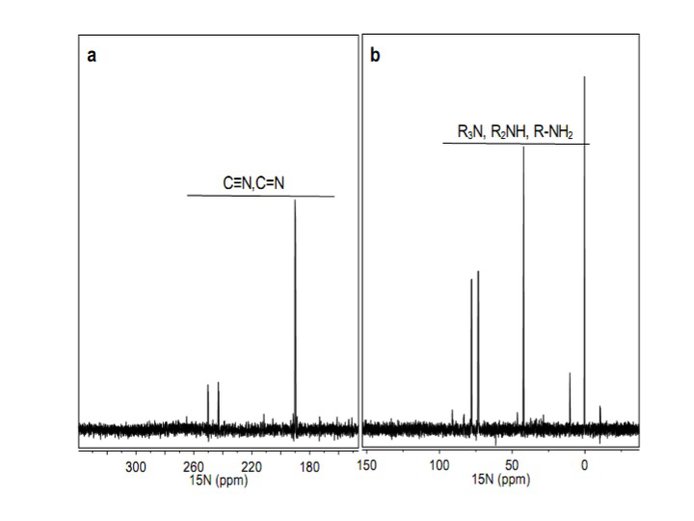
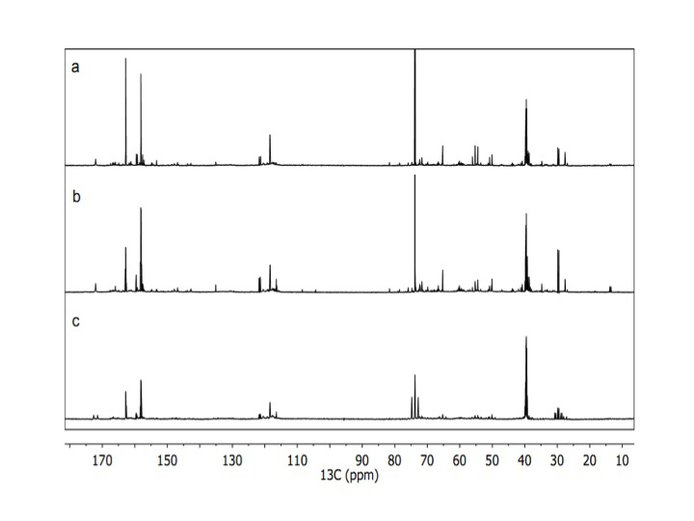
The definition and assessment of future flight capable analytical methods for complex organic analysis was pursued, in particular evaluating the potential of nuclear magnetic resonance (NMR). Initial work has been applied to the analysis of laboratory-produced tholin-like samples using one dimensional NMR techniques. Using 13C and 15N isotope labeled tholins, they have carried out the 1-dimensional (1D) NMR experiments on the three nuclei (1H, 13C and 15N) (Figure 9) and developed 1D decoupling NMR experimental methods to help define a functional group inventory of tholins (Figure 10). The chemical shifts of the 1H, 13C and 15N all point to amine, nitrile, imine and N-heteroaromatic functionality being present in the tholin samples.
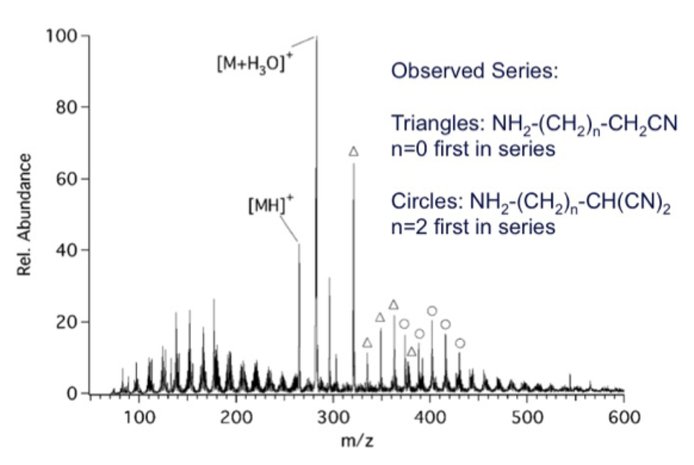
Titan organics comprise a very complex mixture of compounds. Several approaches are being developed in addition to NMR analysis that provide targeted detection of specific functional groups, such as nitriles, imines, primary amines, and carbon-carbon multiple bonds. Experiments for the selective and sensitive detection of primary amines through mass spectrometry are currently being conducted due to the biological prevalence of small molecule (<200Da) primary amines. The use of 18-Crown-6 ether adduct formation in electrospray ionization mass spectrometry (ESI/MS) has shown promise. This allows for the selective detection of primary amine functionality in a complex mixture of organic compounds. As a test of this idea, laboratory produced tholins were complexed with 18-Crown-6. Each tholin was dissolved in dichloromethane at 2 mg/ml with 2mM 18-Crown-6 ether. Adduct formation is shown in Figure 11. The marked peaks in this figure, with M = 18-Crown-6 ether, correspond to two different series (triangles: NH2-(CH2)n-CH2CN, where n=0 is the first in the series, and circles: NH2-(CH2)n-CH(CN)2 ,where n=2 is the first in the series). Complexation with 18-Crown-6 ether led to the identification of primary amine functionality. Ongoing work is now pursing selective detection of primary amine functionality using an amine reactive reagent with a permanent charge.
An open question is: “What chemical structures might support the genetic component of Darwinian evolution in Titan environments?” This is being approach theoretically, including generating hypotheses for biopolymers that might dissolve in, be formed in, and remained stable in two Titan environments, and experimentally, synthesizing versions of those biopolymers and determining their performance in various models for Titan environments. Two possibilities have been explored: (1) polyarsenate biopolymers as genetic materials in a hypothetical subsurface ammonia-water eutectic on Titan, and (2) polyethers as genetic materials in the surface hydrocarbon oceans on Titan.
Thermochemical and dynamic modeling is being used to provide improved constraints on the available chemical energy and trace element fluxes to facilitate potential life on the surface of Titan. Previous published modeling of the energetics of putative metabolic reactions on Titan is being expanded upon to include a larger range of reactions and more realistic surface inventories for a more rigorous analysis of the potential for the chemical environment at the surface to support life. Measurements from the Cassini dust analyzer are being used to model and constrain the rates of infall of trace materials of potential biological significance.
Figure 1: The Titan chamber (right panel) has been used to test a rain drop sensor (right panel) in Titan’s conditions. A pot (arrow 1) of liquid hydrocarbon (methane or ethane) provides drops that fall on the sensor (arrow 2) that is maintained at cold temperature by sitting on a cold plate. The experiment demonstrated that the sensor works at Titan’s cold temperatures.
Figure 2: Zonal-mean temperatures (in K, top row), zonal winds (in m/s, middle row) and mass streamfunctions (in kg/s, bottom row) averaged over 12 Titan days around northern summer (Ls~90°, left column) and winter (Ls~270°, right column) solstice. Positive streamfunction values indicate clockwise rotation.
Figure 3: As in Figure 2 but for northern spring (Ls=0°, left) and fall (Ls=180°, right) equinoxes.
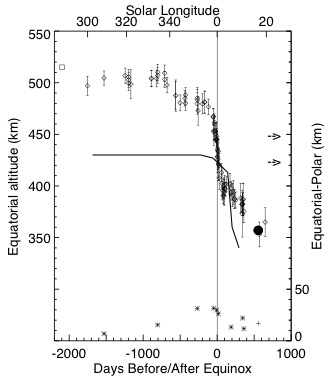
Figure 4. Measurements of Titan’s detached haze seen in Cassini images are shown as a function of time relative to equinox in 2009 (diamonds with error bars). The solid dot is a measurement from Voyager images in 1981 (Rages and Pollack, 1983). The asterisks are equator-pole altitude differences. The solid curve is from the model by Rannou et al. (2002).
Figure 5. Microplasma reactor used to produce tholin-like nanoparticles. (a) Schematic diagram. (b) Particle size distribution measured using a nano radial differential mobility analyzer (Brunelli et al., 2009).
Figure 6. AMS mass spectrum of nanoparticles produced with the microplasma reactor.
Figure 7: Longer wavelength (355 nm followed by 266 nm) laser photo-polymerization product “Titan Tholin” under vacuum (top three pictures), and exposed to atmosphere (bottom left), in methanol solution (bottom right), and the KBr window after cleaning the “UV-photo-tholin” (bottom middle). Color of these polymeric non-volatile organic residue is very similar to Titan’s haze and Titan tholins prepared through other procedures such as discharge.
Figure 8. Fluidized bed reactor; (a) Schematic representation of agitation of beads in fluidized bed; (b) current generation fluidized bed reactor in use; (c) evidence for charging of beads as they stick to walls of reactor through electrostatic interactions.
Figure 9. Solution-state 15N NMR spectra of Titan tholin in DMSO-d6 with 1H decoupling (chemical shift reference of 15N: δN (NH3) =0 ppm): a) 340 to 140 ppm, b) 160 to -40 ppm.
Figure 10. Solution-state 13C NMR spectra of Titan tholin with different decoupling methods: a) with 1H and 15N decoupling, b) with only 1H decoupling and c) without 1H or 15N decoupling.
Figure 11. ESI/MS spectrum of 18-Crown-6 complexed Saker tholins. The triangles and circles indicate complexed species fitting a particular compound series.
Table 1. Gas solubilities in liquid methane and ethane at 94 K. Units are in mole fraction.
Publications
-
Benner, S. A. (2011). Comment on “A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus”. Science, 332(6034), 1149–1149. doi:10.1126/science.1201304
-
Benner, S. A., Kim, H-J., & Yang, Z. (2010). Setting the Stage: The History, Chemistry, and Geobiology behind RNA. Cold Spring Harbor Perspectives in Biology, 4(1), a003541–a003541. doi:10.1101/cshperspect.a003541
-
Choukroun, M., & Sotin, C. (2012). Is Titan’s shape caused by its meteorology and carbon cycle?. Geophysical Research Letters, 39(4), n/a–n/a. doi:10.1029/2011gl050747
-
Kim, H-J., Ricardo, A., Illangkoon, H. I., Kim, M. J., Carrigan, M. A., Frye, F., & Benner, S. A. (2011). Synthesis of Carbohydrates in Mineral-Guided Prebiotic Cycles. Journal of the American Chemical Society, 133(24), 9457–9468. doi:10.1021/ja201769f
-
Newman, C. E., Lee, C., Lian, Y., Richardson, M. I., & Toigo, A. D. (2011). Stratospheric superrotation in the TitanWRF model. Icarus, 213(2), 636–654. doi:10.1016/j.icarus.2011.03.025
-
West, R. A., Balloch, J., Dumont, P., Lavvas, P., Lorenz, R., Rannou, P., … Turtle, E. P. (2011). The evolution of Titan’s detached haze layer near equinox in 2009. Geophysical Research Letters, 38(6), n/a–n/a. doi:10.1029/2011gl046843
- Benner, S.A. (2010). Chemistry, Life, and the Search for Aliens. In: Hoover, R.B., Levin, G.V., Rozanov, A.Y. & Davies, P.C.W. (Eds.). Instruments, Methods, and Missions for Astrobiology Xiii. Vol. 7819.
- Benner, S.A. (2010). Talking about life. In: Impey, C. (Eds.). Conversations on Astrobiology. New York, NY: Cambridge University Press.