2011 Annual Science Report
 NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2010 – AUG 2011
NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2010 – AUG 2011
Survivability of Icy Worlds
Project Summary
Investigation 2 focuses on survivability. As part of our Survivability investigation, we examine the similarities and differences between the abiotic chemistry of planetary ices irradiated with ultraviolet photons (UV), electrons, and ions, and the chemistry of biomolecules exposed to similar conditions. Can the chemical products resulting from these two scenarios be distinguished? Can viable microbes persist after exposure to such conditions? These are motivating questions for our investigation.
Project Progress
Survivability on icy Worlds investigation examines the survivability of biological compounds under simulated icy world surface conditions, and compares the degradation products to abiotically synthesized compounds resulting from the radiation chemistry on icy worlds.
Co-Investigator Murthy Gudipati and his group focused on the spectroscopy of ices and ices containing PAH impurities under UV and electron irradiation, aiming at understanding the chemistry of icy solar system surfaces such as Europa. In particular they have been successful in commissioning MALDI-TOF-MS experiment to investigate water-ices at any given temperature between 5 and 200 K. To the best of our knowledge, there are only two labs that dedicate to study chemistry in water-ice using MALDI-TOF-MS, one of them situated in France and the other Dr. Gudipati’s “ice spectroscopy lab, ISL” at JPL. Preliminary studies on Toluene (C7H8) showed that Tolune, upon Lya irradiation using a hydrogen-flow discharge lamp, undergoes photoionization and subsequent oxidation to form hydroxyl-tolune (or Xylene, C7H7OH), as shown in Figure 2-1.
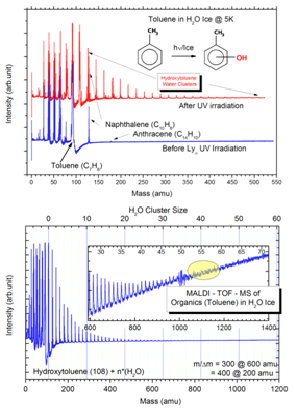
Figure 2-1. MALDI-TOF-MS spectra of organic molecule toluene imbedded in water ice at 5 K. Above: before and after UV-photolysis, clearly seen are the clusters and molecular ion peak of xylene (hydroxylated toluene) at 108 amu. Bottom: enlarged region of the clusters, up to 55 water clusters with xylene are detected.
Very interesting result found in the ISL of Gudipati is that while Toluene, a non-polar hydrocarbon is seen as a single molecule ejected during infrared laser sputtering, Xylene, a polar and OH containing molecule, readily forms hydrogen bonding with water and ice, resulting in sputtering of Xylene water clusters with as high as 50 water molecules in the clusters. These results clearly show that pure hydrocarbons, as soon as they are radiation processed and oxidized, can be strongly bound to the ice, making them less likely to exist as separate “organic phase”.
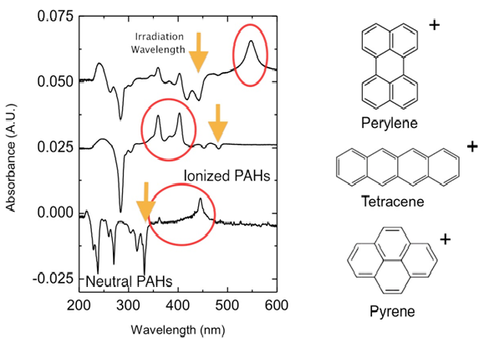
Our ongoing NAI Icy worlds research has shown that PAHs are ionized with low-energy UV-VIS photons, due to lowering of the ionization energy in ice environment. We extended these studies to a variety of PAHs such as Pyrene, Perylene, Tetracene, and Porphyrin (a model biomolecule). The results are summarized in Figure 2-2.
Figure 2-2. Ionization of PAHs Perylene, Tetracene, and Pyrene imbedded in water-ice as impurities – simulating the cometary, and outer solar system icy surfaces. These molecules are ionized with tunable OPO lasers at wavelengths shown by arrows in the figure. The corresponding ionization energies in ice surrounding are at least 2-3 eV below the ionization energies of these molecules in the gas-phase.
We have also found that electrons can damage organic molecules far deeper in ice than electron penetration depths predicted by models. These results are submitted for publication in ApJ. Our preliminary results indicate that electron irradiation of amorphous ices results in photochemical processes in ice itself that could lead to partial “localized” crystallization of amorphous ices. These results are being proven through detailed experimental studies and also being communicated for preliminary publication.
As a part of Investigation 2, Co-Investigators Paul Johnson, Robert Hodyss have continued studying the photolysis of organic molecules in icy matrices relevant to solar system bodies. Their goal is to determine the precise chemical pathways that various molecules evolve in analog solar system ices as a function of temperature and wavelength. This involves photolysis of organics in ice, pure organics, matrix isolated organics, and organics under a layer of peroxide (to provide a source of hydroxyl radicals). Addition baseline spectra are also taken of suspected products to confirm identifications. They are measuring the photolytic lifetimes of hydrocarbons in both water and nitrogen matrices. They are also studying the photolytic chemistry of amino acid (glycine) and lipid (oleic acid and 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphocholine; SLPC) degradation through matrix isolation experiments.
An extensive set of data have been collected on photolysis of glycine, phenylalanine, oleic acid and SLPC. This work has involved overcoming the technical challenge of vapor deposition these large, non-volatile organics from a heated crucible without destruction of the molecule. A paper has recently been submitted to the journal Astrobiology where we report a wavelength resolved study of UV photolysis of matrix-isolated glycine and phenylalanine. In all cases, decarboxylation is identified as the decomposition pathway. Further, we show that as a consequence of the significantly larger solar irradiance at longer wavelengths, it is the longer wavelength UV photons that will dominate amino acid destruction over more energetic, but less abundant, photons on icy bodies. Decarboxylation of amino acids at 254 nm was explicitly demonstrated, further emphasizing the importance of including the entire integrated solar spectral irradiance up to that wavelength (as a minimum) when considering the survivability of amino acids on the surface of icy bodies. Considering solar UV photolysis alone, we cannot reasonably expect to find amino acids in clean surface ices either remotely, or in situ at easily accessible depths. When the effects of other radiation sources such as the solar wind and magnetospheric particles (where applicable) are combined with UV photolysis and radical chemistry, it seems unlikely that organics can survive long enough on the surface of an icy planetary body to be detected without being frequently replenished from a shielded source such as an subsurface ocean.
Photochemical studies of thin cryogenic ice films composed of N2 and CH4 in ratios analogous to those on the surfaces of Neptune’s largest satellite, Triton, and on Pluto have been published in Icarus. Experiments were performed using a hydrogen discharge lamp, which provides an intense source of ultraviolet light in order to elucidate the solar induced photochemistry of these icy bodies. Initial characterization via infrared spectroscopy showed that C2H6, C2H4 and C2H2 are formed by the dissociation of CH4 into H, CH2 and CH3 and the subsequent reaction of these radicals within the ice. Acetylene, C2H2,, is further photodissociated into C2. Other radical species such as C2, C2-, CN, NCN and CNN are observed in the visible and UV regions of the spectrum. These species imply a rich chemistry based on reactions of atomic carbon with the N2 matrix. Ultimately, this work suggests that C2-, CN, and CNN may be found in significant quantities on the surfaces of Triton and Pluto and that new observations of these objects in the appropriate wavelength regions are warranted.
A study of UV stimulated fluorescence and phosphorescence of benzene, toluene and the isomers of xylene in water ice has been published in the journal Astrobiology. The temperature dependence of the fluorescence and phosphorescence intensities was found to be independent of the thermal history and phase of the ice matrix in all cases. All phosphorescent emissions were found to decrease in intensity with increasing temperature. Similar behavior was observed for fluorescence in pure benzene, while the observed fluorescence intensity in water ices was independent of temperature. These results have implications for exploiting such phenomena for in situ detection of organics is solar system ices.
Recent experimental results that are currently being analyzed include:
- The photolytic decomposition of dipicolinic acid is water ice was studied. Dipicolinic acid is a major component of bacterial spores that could serve as a biomarker on icy worlds.
- Stimulated fluorescence of dipicolinic acid in water ice was investigated as a means of informing search strategies on icy worlds.
- Work on UV stimulated fluorescence and phosphorescence of PAHs in water ice is continuing as an extension of our previously published work.
As a part of Investigation 2, Co-I Dalton has demonstrated that surface deposits on Europa contain both hydrated salts, frozen brines and sulfuric acid (Dalton et al., 2011a). This confirms predictions of Carlson et al., (2005) that a radiolytic sulfur cycle is responsible for creation of sulfuric acid hydrate via chemical processing driven by magnetospheric charged particle bombardment. Dalton has now mapped the sulfuric acid distribution using Galileo Near-Infrared Mapping Spectrometer (NIMS) observations, and compared the sulfuric acid abundances with models of electron and ion energy deposited into the surface (Dalton et al., 2011b). The implications of this work suggest that while salts and brines in the surface deposits may be derived from an interior ocean, the sulfuric acid is “printed” over the surface irrespective of the underlying geologic unit. Dalton’s method provides a means of subtracting the sulfuric acid concentration and determining the relative concentrations of the remaining materials, which are linked to oceanic composition; in addition, the sulfuric acid concentration may provide a means of estimating exposure ages for young, disrupted areas. This will be useful in determining landing sites most likely to host viable biomolecules.
Dalton’s analysis of the relationship between exogenically-produced sulfuric acid hydrate, and endogenically-derived hydrated magnesium and sodium sulfates and brines has already proven useful in selection of potential landing sites for a proposed Russian Europa lander mission (Ivanov et al., 2011; Dalton et al., 2011c).
Meanwhile, working with Cassini Visual and Infrared Imaging Spectrometer (VIMS) observations, Dalton has also demonstrated the presence of organic compounds on the Saturnian satellite Hyperion. Dark lag deposits in oddly shaped depressions exhibit spectral signatures indicative of both aromatic and aliphatic hydrocarbons, as seen by Cassini VIMS observations (Dalton et al., 2011d, submitted to Icarus). Additional, weaker signatures suggest the presence of nitrile (C-N bearing) compounds as well.
As a part of Investigation 2, Co‐investigator Cooper and group have been exploring the possibility of oxidant (O2 and H2O2) formation, for the interpretation of both surface and atmospheric composition of icy satellites, due to surface reactions of OH. They have previously deposited OH radicals (with H2O) produced in a gas‐phase discharge of H2O dilute in helium, neon and argon and found significant quantities of H2O2 and O2 are produced. They can quantify the H2O2 abundance in the IR, but they have up until now been unable to quantify OH and O2 abundances.
In the last year they have installed a UV‐vis spectrometer into their system for the purpose of quantifying OH and O2. They have been unable to detect the latter using the UV‐vis and so they are now turning to a mass spectrometric method to determine O2 yield. They have been able to successfully quantify the amount of OH produced in the discharge (using matrix‐isolation) by measuring the 310 nm absorption band of OH. They use this data to calculate an intrinsic band strength (A‐value) of OH in the IR (never before published but will be incorporated into an upcoming manuscript). There does not appear to be enough OH in the discharge to account for the H2O2 being produced so they think there may be a significant amount of O atom produced in the discharge producing H2O2 upon deposition. The group is now working towards refining their technique using a flow reactor to produce OH radicals chemically from the reaction of O3 and H to remove the interfering effects of other species.
In collaboration with researchers at The University of Western Australia, the group has shown that methanol (CH3OH) was produced from the reaction of CH4 and O(1D) by using the matrix isolation technique In the experiments, two streams of gases – CH4:Ar and H2O:Ar (with discharge on) were concurrently deposited onto a 10 K optical window. Using isotopic substitution, it was shown that the dominant reaction channel for methanol formation was that of CH4 reacting with O(1D) (the latter produced in the discharge of H2O:Ar). This reaction channel is mostly ignored in the literature in favor of the CH3 + OH reaction. They are continuing the matrix isolation work and will be extending to ice work in the near future to assess the relative importance of these channels in methanol formation.
A high‐school intern worked over the summer in the lab attempting to synthesize carbonic acid (H2CO3) in order to acquire matrix‐isolated spectra of the molecule. Previous work on carbonic acid has been in ices. Although its identification has now been reported numerous times, a definitive comparison of vibrational spectra to quantum calculations is still required to conclusively identify the elusive molecule. The student built and set up apparatus to do this. In preliminary experiments, it appears carbonic acid decomposes in the transfer line prior to deposition for analysis. This procedure will be refined the next couple of months.
In Investigation 2, Co-Investigator Dr. Gordon Love and his group at the University of California, Riverside, have three major goals: 1) investigate the production, preservation, and detection of chemotrophic lipid biological markers in Arctic thaw lakes as an analog for life detection under Icy Worlds conditions. 2) Generate historical, biomarker-based records of methane cycling and primary production in Arctic permafrost-bound lakes from sediment core analyses. 3) Integrate biomarker records with parallel datasets regarding microbial ecology, water column chemistry and ice composition obtained by other team members.
A fieldwork in October 2010 was conducted to collect sediment core samples which have all been freeze-dried and gently homogenized, completing the first step of the protocol developed for this project. Sedimentological descriptions of the cores have been compiled for communal use. Bulk inorganic geochemical analyses have been conducted on all October samples. Total lipid extracts have been obtained for all samples, and catalytic hydropyrolysis of whole sediment has been conducted on approximately half of the samples. The following describes the group’s field and laboratory techniques.
Sampling: Sediment cores were collected through April 2010 ice cover using a modified piston push-core apparatus (Fisher et al, 1992). Cores were kept refrigerated or partially-frozen until sample processing. Sampling intervals were assigned for individual cores based on changes in visible properties (color, grain size, composition, sedimentary fabric) and to ensure a representative profile of the core. The cores were sectioned into approximately 10 cm intervals and sediment aliquots of 5 to 25 g were removed from the central part of each section. Sediment samples for lipid biomarkers were stored at -20°C and freeze-dried prior to extraction.
Inorganic Geochemical Analyses: Total inorganic carbon (% carbonate) and total organic carbon were determined through subtraction of acid-released CO2 from total carbon determined through combustion. Carbon and oxygen isotopes of carbonate (δ13C, δ18O) detected in the Sukok seep samples was measured through phosphoric acid digestion and continuous flow gas chromatography-isotope ratio mass spectrometry (GC-IRMS) at UCR.
Lipid Extraction and Analysis: Sediment samples (6 to 10 g) were extracted using 9:1 v/v methylene chloride:methanol in a CEM Microwave Accelerated Reaction System (100°C, 15 min) with teflon vessels and a solvent to sample ratio of 7.5 mL g-1 or greater. Total lipid extracts were dried over sodium sulfate and gently blown down under N2. The extracts were separated by silica gel column chromatography into neutral, glycolipid, and phospholipid fractions by successive elution with methylene chloride, acetone and methanol, respectively. Silica gel column chromatography was used to further separate the neutral fraction into hydrocarbon, ketone, and alcohol fractions with hexane, 5% ethyl acetate in hexane and 20% ethyl acetate in hexane, respectively. Separation of total lipid extracts into hydrocarbon, alcohol and acid fractions through solid phase extraction has also been tested on NW and IK samples. S0 was removed from the hydrocarbon fraction using activated copper pellets. An internal standard (d4- ethylcholestane) was added to the hydrocarbon fraction for quantification. The alcohol and acid fractions and an aliquot of total lipid extract from each sample were derivatized (BSTFA+ 1%TMS) for anlaysis via gas chromatography-mass spectrometry (GC-MS).
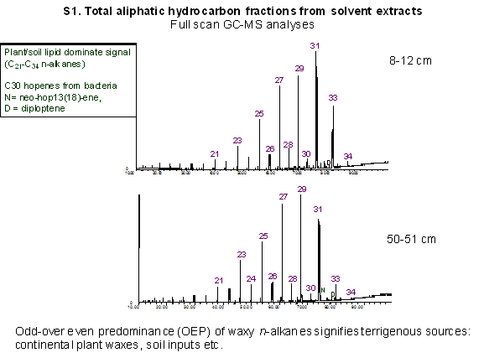
The hydrocarbon fractions obtained from solvent extraction and catalytic hydropyrolysis (HyPy) were analyzed by GC-MS in full scan (monitoring from m/z 50-600 range) or selected ion monitoring mode (Fig. 2-8).
Figure 2-3. Comparison of total free aliphatic hydrocarbon distributions for two sediment horizons from S1 core. Continental plant wax n-alkanes, with a distinct odd-over even carbon number preference (EOP), dominate the overall signal though terrestrial plant n-alkanes are generally selectively enriched compared with shorter chain n-alkanes due to their highly recalcitrant nature.
Additional quantification of specific biomarkers was conducted through metastable reaction monitoring-gas chromatography-mass spectrometry (MRM-GC-MS) on a Waters Autospec Premier instrument. Hydropyrolysis products and hydrocarbon fractions will be analysed by GC-C-IRMS for compound-specific carbon isotope measurements. Methods were developed in-house at UCR to focus on biomarker compounds. Procedural blanks prepared concurrently with samples ensure acceptably low levels of laboratory contamination.
Hydropyrolysis: Catalytic hydropyrolysis of unextracted, freeze-dried sediments was performed to convert functionalized lipids into readily-analyzable hydrocarbon (aliphatic) form for GC-MS analysis. Unextracted sediment samples were loaded with 3 wt% sulfided molybdenum catalyst prior to standard hydropyrolysis heating (150 bar H2, 5 L min-1, temperature program: 30°C to 250°C at 100°C min-1, 250°C to 525°C at 8 °;C min-1). Products were collected on 2 g 35-70 silica in a dry ice-cooled trap and separated into aliphatic, aromatic, and NSO fractions by elution with hexane, methylene chloride:hexane, and methanol, respectively. The aliphatic fractions were then analyzed in detail using conventional GC-MS and MRM-GC-MS as described above.
Results:
i) Free (extractable) hydrocarbons released from Sukok seep S1 core (to a depth of 51 cm), Sukok center site (S3), Ikroavik (IK), and North of Walakpa (NW) lakes show significant source contributions from continental plant waxes, algae and bacteria but no strong signals unambiguously identifying large inputs of archaea (for example, methanogens or anaerobic methanotrophs) although trace amounts of biphytane (C40 acyclic ioprenoidal archaeal marker) are present in some samples. Minor amounts of ancient mature hopane and sterane biomarkers were detected in S1, indicating a very small petroleum-like contamination, but most of the hydrocarbons were immature and derived from lake organisms (or plant matter transported into the lake) which were deposited and incorporated into sediments.
ii) Catalytic hydropyrolysis experiments have been conducted on whole S1, Sukok seep (SS) core, S3, IK, and NW sediments to convert functionalized lipids (alcohols, acids, etc.) to GC-amenable hydrocarbons. Analysis of HyPy products supported source inputs from algae (dominated by green algae), continental plant waxes and bacteria, with trace evidence for archaeal inputs (biphytane) in some samples. In terms of methane cycling microbes, trace amounts of 3b-methylhopanes were apparent from MRM-GC-MS analyses of S1; these were likely derived from microaerophilicType I methanotrophic proteobacteria which most probably lived in the water column and used molecular oxygen (O2) to oxidize methane.
iii) Total lipid extracts from all samples as well as alcohol and acid fractions from IK and NW, were subjected to BSTFA derivatization in order to further investigate the compostion of organic matter in the lakes. Derivatized samples also reflect significant inputs from land plants and algae (sterols), and bacteria, but little obvious contribution from archaeal lipids.
iv) Unpublished results from Katey Walter Anthony indicate that the methane source emanating from Sukok seep (S1, SS) site is actually radiocarbon-dead (>60,000 years old) and probably derived from coalbed methane. Thus, it is not surprising that no abundant and characteristic lipid signatures from archaea could be obtained from this S1 sediment core.
v) δ13C analyses of Sukok seep carbonate yields only slightly negative values (-3.8 to -2.4 per mil) suggesting a detrital rather than methane-oxidation source of deposited carbonate. Sediment composition (grain size, TOC, % carbonate) indicate variable inter- and intra-lake depositional conditions or inputs. Comparison of these sediment properties with lipid biomarkers and molecular microbiological results will constrain the impact of fundamental sediment properties on microbial communities.
vi) The relative scarcity of archaeal lipids in hydropyrolysis products from whole sediments indicates that archaeal biomass is extremely low relative to other sources of organic matter, despite detection of methane and molecular evidence from Paula Mattheus (graduate student, DRI) for archaea, including methanogens, in the sediment pores. This raises questions regarding metabolic rates of methane production and oxidation, including the relative importances of temperature and available substrate (e.g. lack of sulfate for anaerobic oxidation of methane). If methane in the center of Sukok Lake, the lake north of Walakpa, and Ikroavik Lake is sourced from present-day methanogenesis as hypothesized, the per-cell rate of methane production must be high enough to accomodate both the concentration of methane present and the low archaeal biomass. Alternatively, slow methane production by scarce archaea may put limits on the “catchment” size required to supply observed methane fluxes.
As a part of Investigation 2, Co-I, Adrian Ponce carried out the following three tasks which will be described below:
Task 1: Viability Assessment of Microbes in Simulated Europan Radiation Environment
This task focuses on the viability of spores and radiation resistant microbes in ices under radiation environment. Planetary ice sheets are likely depositories of putative microbial extant/extinct life from the potential biospheres on, for example, Mars, Europa, and Enceladus. Hardy microorganisms embedded within these ices would have the potential to survive over millions of years. Experiments require the preparation of uniform monolayer distributions of spores and resistant microbes on aluminum targets for radiation inactivation experiments performed by Co-I Kevin Hand at temperature regimes of the Europa surface. Viability assessment of spores and microbes after irradiation are performed by culture, germinability, and ATP assays.
Status: After one round of sample preparation, irradiation, and viability assessment a number of challenges in sample preparation were identified that need to be addressed before inactivation parameters can be obtained. Specifically, deposition of uniform monolayers in the target area of the electron beam has been difficult to obtain. At this point, we have evaluated numerous strategies, including various detergents and drying conditions, and evaluated their suitability with electron microscopy. We estimate that irradiation experiments will be possible in the last quarter of 2011, and that subsequent data collection will proceed on target for completing this task on time and on budget.
Task 2: Viability of Microbes Embedded in Kilimanjaro’s Glacial Ice
The summit of Kilimanjaro in equatorial East Africa hosts melting/subliming glaciers with stratigraphic layering, including embedded atmospheric dust, that are believed to be nearly 12,000 years old. The periglacial soils and supraglacial meltwater ponds are among the most extreme microbial habitats on Earth, as microbes eking out a living in these near-sterile environments face extreme diurnal freeze-thaw cycles, high UV flux, half an atmosphere of pressure, and extreme low organic carbon content. The Kilimanjaro summit also contains fissure vents and fumaroles that afford the possibility of investigating microbial habitability, in terms of viability, diversity and abundance, across temperature and water gradients that represent a continuum of microenvironments. These factors make Kilimanjaro an excellent Mars analog that will further our understanding of microbial growth boundary conditions, habitability, and preservation of organics.
Status: To date, there are no investigations published on the microbiology of Kilimanjaro glaciers. We aseptically collected near basal ice from a vertical glacial cliff of the Northern Ice Field, and filtered particulate matter from eight ice layers, including two layers that contained visible accumulations of dust. Microbial diversity data show that at the time of dust layer formation, the glacier surface hosted an active microbial, cold-water ecosystem. We found that a majority of bacterial clones, as determined by bacterial 16S rRNA gene sequencing, are most closely related to those isolated from cold water environments. This is further supported by the observation of a large mud-rich pond on the surface of NIF in 2008, which likely represents conditions present when the ice dust layers formed. We anticipate that future investigations of this tropical-alpine, supraglacial, cold-water ecosystem will yield insights into microbial activity at the temperature, water and organic carbon limits for life on Earth.
Task 3: High Pressure Microbiology
The emerging field of high pressure microbiology is relevant to the largest segment of Earth’s biosphere, as 95% of water containing 55% of all waterborne microbes is located more than 200 m (i.e., >2 MPa) below sea level (i.e., 0.1 MPa). On Europa the fraction of water volume at high hydrostatic pressure is expected to be even greater. A growing body of literature documents investigations of high-pressure physiology of piezophiles and non-piezophiles, and their adaptations to extreme high-pressure environments. However, much less is known about microbial adaption to high pressure versus effects of temperature, pH and osmotic pressure.
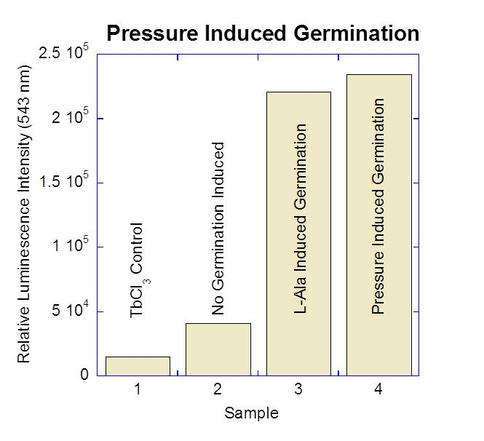
Bacterial spores are the most resilient and long lived microbial forms, with report of longevity up to several hundred million years, making bacterial spores the most likely organisms to survive an interplanetary journey. Once embedded into Europan ice and subdued into its ocean by convection, the possibility of germination and outgrowth remains plausible. Recently, we and others have shown that aerobic Bacillus spores germinate as a result of being exposed to high hydrostatic pressures (50-800 MPa) (Figure 2-4). However, this organism is aerobic, and it is expected that the Europan ocean will be anoxic. Here we propose to investigate the effects of high hydrostatic pressure on the germination of Clostridium sporogenes and C. frigoris spores, which are anaerobic species and the latter one also being psychrophilic (i.e., cold loving). Germination can be induced at high pressures (100 mPa).
Figure 2-4. Preliminary results from Ponce lab. Germination can be induced at high pressures (100 mPa).
References:
Fisher, M. M., M. Brenner, K. R. Reddy (1992) A simple, inexpensive piston corer for collecting undisturbed sediment/water interface profiles. Journal of Paleolimnology 7, 157-161
Publications
-
Cooper, P. D. (2012). Oxygen in the Outer Solar System. Journal of Chemical Education, 89(2), 181–182. doi:10.1021/ed200013q
-
Dalton, J. B., Shirley, J. H., & Kamp, L. W. (2012). Europa’s icy bright plains and dark linea: Exogenic and endogenic contributions to composition and surface properties. Journal of Geophysical Research: Planets, 117(E3), n/a–n/a. doi:10.1029/2011je003909
-
Garozzo, M., Fulvio, D., Kanuchova, Z., Palumbo, M. E., & Strazzulla, G. (2009). The fate of S-bearing species after ion irradiation of interstellar icy grain mantles. A&A, 509, A67. doi:10.1051/0004-6361/200913040
-
Hodyss, R., Howard, H. R., Johnson, P. V., Goguen, J. D., & Kanik, I. (2011). Formation of radical species in photolyzed CH4:N2 ices. Icarus, 214(2), 748–753. doi:10.1016/j.icarus.2011.05.023
-
Ivanov, M. A., Prockter, L. M., & Dalton, B. (2011). Landforms of Europa and selection of landing sites. Advances in Space Research, 48(4), 661–677. doi:10.1016/j.asr.2011.05.016
-
Johnson, P. V., Hodyss, R., Bolser, D. K., Bhartia, R., Lane, A. L., & Kanik, I. (2011). Ultraviolet-Stimulated Fluorescence and Phosphorescence of Aromatic Hydrocarbons in Water Ice. Astrobiology, 11(2), 151–156. doi:10.1089/ast.2010.0568
-
Johnson, P. V., Hodyss, R., Chernow, V. F., Lipscomb, D. M., & Goguen, J. D. (2012). Ultraviolet photolysis of amino acids on the surface of icy Solar System bodies. Icarus, 221(2), 800–805. doi:10.1016/j.icarus.2012.09.005
-
Modica, P., & Palumbo, M. E. (2010). Formation of methyl formate after cosmic ion irradiation of icy grain mantles. A&A, 519, A22. doi:10.1051/0004-6361/201014101
-
Palumbo, M. E., Baratta, G. A., Leto, G., & Strazzulla, G. (2010). H bonds in astrophysical ices. Journal of Molecular Structure, 972(1-3), 64–67. doi:10.1016/j.molstruc.2009.12.017
-
Pearce, M. P., Bussemaker, M. J., Cooper, P. D., Lapere, K. M., Wild, D. A., & McKinley, A. J. (2012). Formation of methanol from methane and water in an electrical discharge. Physical Chemistry Chemical Physics, 14(10), 3444. doi:10.1039/c2cp22135g
- (2011). Astrobiology of Icy Worlds: Photochemistry of Planetary Ices. Physics Colloquium (MH 606). California State University, Fullerton, California.
- Cooper, P.D., Do, N., Strube, A. & Ammann, L.M. (2010). Oxidant Loading of Icy Satellite Surfaces by Re-deposition and Subsequent Surface Chemistry. DPS. Pasadena CA.
- Dalton, I.J.B., Shirley, J.H., Cassidy, T., Paranicas, C.J. & Kamp, L.W. (2011b). Surface effects of endogenic and exogenic processes on Europa. EPSC-DPS Joint Meeting, 6(649).
- Dalton, J.B., Prockter, L.M. & Ivanov, M.A. (2011c). Surface materials on Europa: Considerations for Landing Site Selection. Proc. 2nd Moscow Solar System Symposium (2M-S3). Moscow.
- Do, N., Strube, A., Ammann, L.M. & Cooper, P.D. (2011, Submitted). A Laboratory Investigation of the Chemistry of Resurfacing on Icy Bodies. Ap. J.
- Hodyss, R. (2011). New Horizon Workshop on Icy Surface Processes. Flagstaff Arizona.
- Hodyss, R., Howard, H.R., Johnson, P.V., Goguen, J.D. & Kanik, I. (To Be Submitted). Atomic Carbon Chemistry in Photolyzed Triton-Like Ices. Icarus.
- Hodyss, R., Kim, H.I., Bolser, D.K., Johnson, P.V., Goguen, J.D. & Kanik, I. (To Be Sbmitted). Ultraviolet Photolysis of Lipids. Icarus.
- Johnson, P. (2010). Atomic Carbon Chemistry in Photolyzed Triton-like Ices. 2010 Fall AGU Meeting. San Francisco, CA.
- Johnson, P.V., Hodyss, R., Howard, H.R., Goguen, J.D. & Kanik, I. (2010). Atomic Carbon Chemistry in Photolyzed Triton-like Ices. 42nd annual meeting of the Division for Planetary Sciences of the American Astronomical Society. Pasadena, CA.
- Pearce, M., McKinley, A.J. & Cooper, P.D. (In Preparation). Formation of Methanol in Argon Matrices. Chem. Phys. Phys. Chem.
- StrazStrazzulla, G. Ion Bombardment of astrophysical iceszulla, G. Ion Bombardment of astrophysical ices. 24th International Conference on atomic collision in solids. Cracow, Poland.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Robert Hodyss
Project Investigator
Louis Allamandola
Collaborator
Bob Carlson
Collaborator
Giovanni Strazzulla
Collaborator
-
RELATED OBJECTIVES:
Objective 2.2
Outer Solar System exploration
Objective 3.2
Origins and evolution of functional biomolecules
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.3
Biochemical adaptation to extreme environments
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems