2011 Annual Science Report
 NASA Goddard Space Flight Center
Reporting | SEP 2010 – AUG 2011
NASA Goddard Space Flight Center
Reporting | SEP 2010 – AUG 2011
Cosmic Ice Laboratory Progress Report
Project Summary
Scientists at the Cosmic Ice Laboratory with the Goddard Center for Astrobiology study the formation and stability of molecules under conditions found in outer space. During the past year, studies of amino-acid destruction were continued with a manuscript in preparation. Projects on sulfuric-acid hydrates were completed, and a new project involving thermal chemistry at Europa-like temperatures was begun. All of this work is part of the Comic Ice Laboratory’s continuing contributions toward understanding the chemistry of biologically-related molecules and chemical reactions in extraterrestrial environments.
Project Progress
Our research focuses on chemical reactions and physical properties of icy materials in extraterrestrial environments dominated by low-temperature and low-pressure conditions. Ionizing radiation sources are used to simulate space radiation conditions and to drive reactions. Infrared (IR) spectroscopy is used to monitor the chemistry in situ within a high-vacuum chamber. Although we traditionally have used 1-MeV protons to initiate ice chemistry, a H2-discharge far-UV lamp, a xenon discharge lamp, and a 10-keV electron source are also available to us. With these tools we can investigate a wide variety of interstellar, circumstellar, and solar-system chemical problems.
Over the past year we have continued our work on amino-acid chemistry, moving away from molecular formation to molecular destruction. We have also completed and published a study of the radiation chemistry of sulfuric acid hydrates as it relates to the surface of Europa.
Amino Acid Destruction by Radiolysis
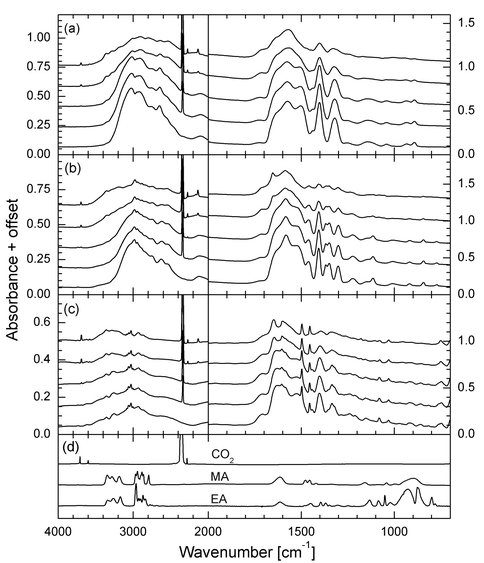
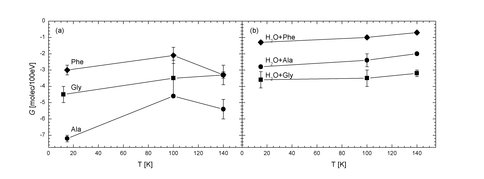
Previous papers from this laboratory have reported on both the radiolytic and photolytic destruction of small molecules known to be in the interstellar medium. Recently, we have turned our attention to similar measurements on amino acids as a preliminary step toward examining even larger species. With NAI support, we have expanded on our previous year’s work in this area to measure the radiolytic lifetimes of the amino acids glycine, alanine, and phenylalanine at three temperatures, both in the pure solid state and in the presence of water-ice. Our long-term goal for this work is to develop a set of rate data for the radiolytic destruction of selected amino acids in various astronomical environments, such as on Titan and in the interstellar medium. Figure 1 contains the spectra of the pure amino-acid samples during irradiation, compared to the spectra of the identified decomposition products CO2, methylamine, and ethylamine. The number of molecules per 100 eV of energy absorbed for the pure samples and the samples mixed with H2O are shown in Figure 2, showing a weak dependence on the temperature of the sample.
Figure 1. Infrared spectra of amino-acid samples during irradiation at 15 K: (a) glycine, (b) L alanine, and© L phenylalanine. In each case spectra are shown after fluences of 0, 5.0 × 1013, 1.0 × 1014, 4.0 × 1014, and 8.0 × 1014 p+ cm 2 (from bottom to top). Panel (d) contains the 15-K absorbance spectra of CO2, methylamine (MA), and ethylamine (EA) films for reference
Figure 2. G-values (in molecules per 100 eV absorbed) for the decays of amino acids in the studied samples versus the substrate temperature (in K) during proton irradiation: (a) in pure samples; (b) in H2O mixtures. Squares: glycine; circles: L-alanine, diamonds: L-phenylalanine.
Radiolysis of Sulfuric-Acid Hydrates
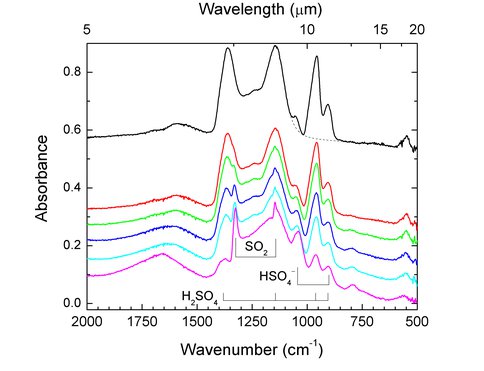
With an emphasis on the radiation chemistry within the surface ices of Europa, we have performed laboratory studies on the 0.8 MeV proton irradiation of ices composed of sulfuric acid (H2SO4), sulfuric acid monohydrate (H2SO4·H2O), and sulfuric acid tetrahydrate (H2SO4·4H2O) between 10 and 180 K. We have identified the main radiation products as H2O, SO2, (S2O3)x, H3O+, HSO4–, and SO42–. Figure 3 shows the spectrum of amorphous sulfuric acid during the course of proton irradiation at 86 K. At high radiation doses, we find that H2SO4 molecules are destroyed completely and that H2SO4·H2O is formed on subsequent warming. This hydrate is significantly more stable to radiolytic destruction than pure H2SO4, falling to an equilibrium relative abundance of 50% of its original value on prolonged irradiation.
Figure 3. Infrared spectra of amorphous H2SO4 during irradiation at 86 K with 0.8-MeV protons. Spectra from top to bottom are after 0, 0.14, 0.29, 0.54, 0.73, and 1.5 × 1015 H+ cm 2.
Extrapolating our results to Europa’s surface, we speculate that the variations in SO2 concentrations observed in the chaotic terrains are a result of radiation processing of lower hydration states of sulfuric acid and that the monohydrate will remain stable on the surface over geological times, while the tetrahydrate will remain stable in the warmer regions but be destroyed in the colder regions, unless it can be reformed by other processes, such as thermal reactions induced by diurnal cycling. This work was done with support from the Goddard Center for Astrobiology as well as the Planetary Geology & Geophysics program.
Other Work
In the past year, our paper on the IR optical constants of nitriles related to Titan has appeared in print, and we have continued our work to measure even more organic compounds. Along these lines, much of the focus of the past year has been to determine the refractive index of hydrocarbons at visible wavelengths, a necessary first step in the process of determining their optical constants at IR wavelengths.
We also have continued our work on oxidants, particularly H2O2, with an eye toward Europa ices. We are also studying the possibility that thermal reactions between H2O2 and organics might take place in ices even in the absence of ionizing radiation. This work is an astrobiological extension to that supported by our Planetary Geology and Geophysics award.
Publications
-
Loeffler, M. J., & Hudson, R. L. (2010). Thermally-induced chemistry and the Jovian icy satellites: A laboratory study of the formation of sulfur oxyanions. Geophysical Research Letters, 37(19), n/a–n/a. doi:10.1029/2010gl044553
-
Loeffler, M. J., Hudson, R. L., Moore, M. H., & Carlson, R. W. (2011). Radiolysis of sulfuric acid, sulfuric acid monohydrate, and sulfuric acid tetrahydrate and its relevance to Europa. Icarus, 215(1), 370–380. doi:10.1016/j.icarus.2011.06.008
-
Moore, M. H., Ferrante, R. F., James Moore, W., & Hudson, R. (2010). INFRARED SPECTRA AND OPTICAL CONSTANTS OF NITRILE ICES RELEVANT TO TITAN’s ATMOSPHERE. The Astrophysical Journal Supplement Series, 191(1), 96–112. doi:10.1088/0067-0049/191/1/96
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Perry Gerakines
Co-Investigator
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules