2011 Annual Science Report
 Georgia Institute of Technology
Reporting | SEP 2010 – AUG 2011
Georgia Institute of Technology
Reporting | SEP 2010 – AUG 2011
In Vivo Deconstruction and Restoration of Ribosomal
Project Summary
We are employing the yeast-three hybrid system to investigate in vivo interactions between a-RNA and ribosomal proteins. Our results demonstrate that a-RNA-γ binds in vivo to L2, L3, L4, L15, and L22. L2 is an initial protein in ribosomal assembly and binds to the intact 23S rRNA independently of other r-proteins. We are currently examining the potential for greater binding in vivo of L3, L4, L15, and L22 to the a-RNA-γ with co-expression of L2 in a yeast hybrid system.
Project Progress
Comprised of both RNA and protein, the ribosome is a molecular fossil with a historical imprint in extant biochemistry. Our goal is to reconstruct an in vivo model of an ancestral ribosome from extant biology and to resurrect a minimal operational structure that could have allowed LUCA to catalyze life’s first peptides and propel the selection process that continues today.
While the ribosomal proteins stabilize the assembly structure, it is the function of the rRNA at the peptidyl transferase center (PTC) to actually perform the catalysis of peptide bond formation during protein synthesis. Positioned in the core of the ribosome, the ancient rRNA of the highly conserved PTC is rooted in the RNA-World. Based on a consensus of ribosomal evolution hypotheses, the Ribo Evo Center has designed and synthesized model for an ancestral peptidyl transferase center, the a-PTC, derived from Thermus thermophilus 23S rRNA plus fragments of certain ribosomal proteins (Hsiao et al in progress). The a-PTC was designed to allow modifications to refine function and analyze the importance of specific 23SrRNA subdomains, as well as helix and stem-loop moieties. The a-PTC was engineered and experimentally demonstrated to assemble in vitro.
The yeast three-hybrid system (Y3H) (1, 2) allows in vivo assessment of RNA-protein interactions. We gave used the Y3H system to study assembly of a-PTC-γ in vivo and and to developed a plateform for high through-put analysis and refinements of a-PTC. Two iterations of the a-RNA model – a-RNA-β2 and a-RNA-γ – have been tested for interaction with ribosomal proteins L2, L3, L4, L15, and L22 in the yeast three-hybrid system. Based on proximity in three dimensional space of the intact ribosome, a-RNA-γ was predicted to associate with L2, L3, L4, L15 and L22.
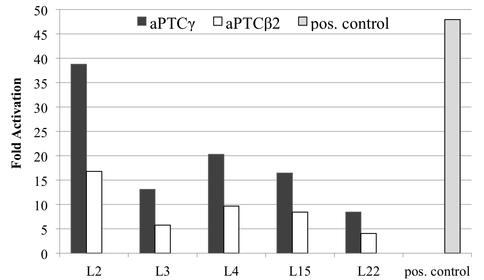
Our initial results suggested an improvement from a-RNA-β2 to a-RNA-γ that resulted in increased in vivo binding with the tested rProteins (Figure 1). L2 is a necessary rProtein in the initial stages of ribosomal assembly (3) and can bind to the rRNA independently of other rProteins. Our data indicates that L2 has the greatest affinity with both a-RNA models, suggesting that the a-RNA recruits rProteins in a trend similar to the intact 23S. We predict that co-expression of L2 will increase the binding of other rProteins to the a-RNA-γ. Currently, we are testing this prediction in a yeast four-hybrid system, in which a-PTC-γ interactions with rProteins L3, L4, L15 and L22 in the presence of L2.
References
1. Hook B, Bernstein D, Zhang B, Wickens M (2005) RNA-Protein Interactions in the Yeast Three-Hybrid System: Affinity, Sensitivity, and Enhanced Library Screening. RNA 11:227-233.
2. Riley KJ, Cassiday LA, Kumar A, Maher LJ, 3rd (2006) Recognition of RNA by the P53 Tumor Suppressor Protein in the Yeast Three-Hybrid System. RNA 12:620-630.
3. Fox GE, Ashinikumar KN (2004) The Evolutionary History of the Translation Machinery. The Genetic Code and the Origin of Life, ed de Pouplana LR (Kluwer Academic / Plenum Publishers, New York ), pp 92-105.
In vivo binding of a-RNA-γ to ribosomal proteins in a yeast three hybrid system. Fold activation calculated from the positive signal over background. Positive control is the interaction of RNA aptamer α-p50 with tumor suppressor protein p532.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Bahareh Azizi
Collaborator
Dana Cook-Schneider
Postdoc
Brande Jones
Postdoc
Eric Anderson
Research Staff
Po-Yu Fang
Graduate Student
Poorna Roy
Graduate Student
Chris Hosford
Undergraduate Student
Andrew St. James
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.2
Production of complex life.