2011 Annual Science Report
 Carnegie Institution of Washington
Reporting | SEP 2010 – AUG 2011
Carnegie Institution of Washington
Reporting | SEP 2010 – AUG 2011
Project 5: Geological-Biological Interactions
Project Summary
This project involves multiple researchers exploring life in extreme environments, the signature of life (chemical, isotopic and mineralogical), and the adaption of life. All together the many sub-topics of this project seek to inform us about where to search for life on other worlds and how to seek evidence that life once existed on other worlds.
Project Progress
Project 5: Geological-Biological Interactions
The projects reported within “Geological-Biological Interactions” section fit several goals of the Astrobiology Roadmap, particularly:
Goal 2: Explore for past or present habitable environments, prebiotic chemistry and signs of life elsewhere in our Solar System.
Goal 3: Understand how life emerges from cosmic and planetary precursors.
Goal 4: Understand how past life on Earth interacted with its changing planetary and Solar System environment.
Goal 5: Understand the evolutionary mechanisms and environmental limits of life.
Goal 7: Determine how to recognize signatures of life on other worlds and on early Earth.
One of the interests is testing the hypothesis that plate tectonics, past or present, is an essential feature of habitable planets that could support life, as we know it. For example, there is isotopic and modeling evidence that hydrogen, produced abiotically in hydrothermal systems, may have been the source of energy for the earliest microbial communities on Earth. It would follow that present day microbial communities that thrive on hydrogen, such as found at Lost City (see 5.1.1 and 5.1.3 chapters) or organisms studied through the experiments for characterization of hydrogen isotope fractionations (see 5.3.5), may be useful model systems for studying early life (Goals 3, 4 and 5).
Likewise, elucidating the carbon, nitrogen and sulfur pathways in a range of environments (i.e. anaerobic, hyperthermophilic vent microorganisms, sulfate-rich aeolian arid environments, modern lake and marsh settings, a range of Paleoproterozoic rocks, as well as using laboratory experimental set up) (see chapters 5.1.1 to 5.1.3, 5.1.5, and 5.3 chapters) could provide insights in the evolution of metabolic networks while providing a model of an ancient microbial community for testing hypothesis (Goals 3, 4 and 5). These studies could also result in the identification of bio-signatures that could be applicable to our search for evidence of hydrothermal systems on other planets and help identify the habitable zones on Mars (see chapters 5.1, 5.2, and 5.3) (Goals 2 and 7).
5.1 Life at the Extremes
There are several teams involved in a number of research projects conducted under this topic.
The John A. Baross group at University of Washington continues to focus on microbial ecology in the extreme environments associated with deep-sea hydrothermal systems and particularly on sub-surface environments associated with hydrothermal systems. The team working on this effort consists of: Rika Anderson (PhD student in Oceanography and Astrobiology), Aditya Chopra (PhD student in Astronomy, Australian National University (JAB as co-advisor)), Billy Brazelton (NAI post-doctoral fellow; co-advised by Matt Schrenk and JAB), Aaron Goldman (NAI post-doctoral fellow at Princeton (JAB as co-advisor)), Florence van Tulder (undergraduate student in oceanography), Stephen Jensen (undergraduate student in biochemistry), Anne Doubllday (undergraduate student). The major research directions in Baross laboratory are 1) the identification and characterization of microbial communities from subseafloor, sulfide and carbonate chimney hydrothermal vent environments and particularly those that form biofilms and use either hydrogen or abiotically produced organic compounds such as formate and acetate as the primary energy sources, and 2) microbial/mineral association including high-temperature biofilm formation, mineral catalysis, and microbial dissolution of minerals as a mechanism for nutrient acquisition. The research projects overlap closely with research of other investigators who are part of the Carnegie NAI team including Matt Schrenk (subsurface biofilm microbial communities and extreme environments), George Cody (ancient metabolic networks and the origin of life), Bob Hazen (mineral catalysis), and Andrew Steele (extreme environments and biosignatures). J. Baross is also affiliated with the NAI Icy Moons team at JPL and the VPL team at the University of Washington in the capacity of providing expertise on hydrothermal systems, microbial ecology, geo-microbiology and the evolution of metabolic pathways over the course of Earth history. Regarding future field expeditions, J. Baross has a proposal pending to return to Lost City hydrothermal vents on the Mid Atlantic Ridge in 2012 or 2013. The main focus will be to better understand the metabolic diversity of a single-phylum archaeal community that better resembles a multi-species community.
The Schrenk Lab at East Carolina University continues to study various aspects of life in extreme environments using microbiological and geochemical approaches; focusing specifically upon: 1) highly alkaline ecosystems associated with serpentinization and 2) high temperature, deep-sea ecosystems. High pH (>11) fluids and associated rocks were collected from serpentinites associated with the Tablelands Ophiolite (Newfoundland, Canada) in Summer 2011, from the Coast Range Ophiolite (California) in April and August 2011, and from the Ligurian Springs (Italy) in October 2010. During each of these field programs, samples were collected for genomic and metagenomic studies of microbial communities and for cultivation experiments, linked to a range of contextual geochemical data provided by our collaborators. For the Tablelands Ophiolite, we worked closely with Penny Morrill at Memorial University of Newfoundland, who is funded by the Canadian Space Agency to study the Tablelands as a Mars analog environment. NAI post-doc Billy Brazelton and Candice Ratley, a Master’s student at ECU traveled to the Tablelands to participate in the field expedition. PI Schrenk traveled to the Coast Range Ophiolite (CRO) at McLaughlin Natural Reserve, near Lower Lake, California, twice during the reporting period to participate in a NAI-sponsored project to obtain drill core samples directly from the serpentinite subsurface. Collaborators on the project include Dawn Cardace (University of Rhode Island), Tori Hoehler (NASA-ARC), and Tom McCollom (U. Colorado). A graduate (Bridget Nelson) and undergraduate student (Sarah Chowdhury) from ECU traveled to CRO to participate in the two-week long drilling project. The CRO drilling project yielded the first samples directly obtained from the serpentinite subsurface environment. Post-doc Brazelton and B. Nelson also traveled to the Ligurian Springs in October 2010 to sample serpentinite seeps with collaborators from ETH-Zurich (G. Früh-Green and S. Lang). In each case, samples are being analyzed for microbial phylogenetic diversity using SSU rRNA genes, functional gene abundance and diversity, and ultimately their connections to the geochemical environment. Furthermore, novel alkaliphilic cultures were obtained from each of these systems and are being used to study microbial geochemistry and to make genomic comparisons.
Additionally, during the reporting period, the Schrenk lab continued their studies of microbial diversity and activities in high temperature, deep-sea hydrothermal ecosystems. These efforts draw upon more than a decade of archived samples collected by collaborators at the University of Washington (D. Kelley and J. Baross). ECU Master’s student Heather Blumenfeld completed her thesis work studying the phylogeny and distribution of genes related to autotrophy in July 2010, and is currently preparing her work for publication.
The CIW team studying life at extremes is a combination of M.L. Fogel and A. Steele’s groups, the team includes: V. Starke (PhD student at CIW and University of Maryland), J. VIdonish (student intern, University of Chicago), J. Kirshtein (USGS, Reston, VA) and M. Glamoclija (research scientist, CIW). The Fogel / Steele group has been continuing their work on Arctic glacial and associated environments from Svalbard (Norway), focusing over the last year on pigment signature tracing and stable isotope analysis of 13C and 15N. This work is important for understanding the nature and origin of life in both modern and ancient ice. Further they are continuing their investigations of microbial ecology of Arctic thermal springs to decipher how variations in environmental conditions affect microbial community makeup. They are using molecular biology tools (16S and 454 sequences and ARISA data) to analyze samples collected over several years. During the last year the collected data have been processed and some of the analyzed data are in preparation for publications. The continuing is also work on gypsiferous arid desert environments from White Sands National Monument from New Mexico. This year focus was on high-resolution mineral analyses and their correlation to molecular and chemical data. The preparation of publications is in progress.
5.1.1 Sub-Seafloor Interactions
Astrobiology research objective of the Baross laboratory continues to focus on microbial ecology in the extreme environments associated with deep-sea hydrothermal systems and particularly on sub-surface environments associated with hydrothermal systems Amaral-Zettler et al., 2010; Baross and Impey, 2011; Walter et al., 2011. Specifically they are focusing on viral communities, their microbial hosts and their role in evolution through horizontal gene transfer at magma-hosted sub-surface hydrothermal systems and the geo-microbiology of peridotite-hosted environments such as Lost City on the mid-Atlantic Ridge Anderson et al., 2011b. The three components of their research include 1) The molecular and physiological characterization of anaerobic microbial communities, including biofilms, that use hydrogen and/or methane and other abiotically produced carbon compounds such as formate and acetate, as their primary energy source(s) W. J. Brazelton and Baross, 2010a; W. J. Brazelton et al., 2010b; Kaye et al., 2011, 2) the possible role of these hydrogen and C1 and C2 metabolizing biofilms in understanding early Earth microbial communities and the origin of multicellularity, and 3) the characterization of the virus communities associated with hydrothermal sub-surface vent environments and their possible role in horizontal gene transfer.
The Schrenk lab at ECU continued to explore the distribution, diversity, and abundance of microorganisms within the walls of deep-sea hydrothermal vent chimneys. Undergraduate Hilary Conrad refined methods to image and quantify microbial cells within chimney walls as part of her independent study project, which was presented locally at ECU research week in April 2011. Master’s student Heather Blumenfeld completed her thesis studying the distribution, abundance, and phylogeny of genes related to carbon fixation from hydrothermal chimney ecosystems. She presented her work as a poster at the Fall AGU meeting in San Francisco in December 2010. Finally, Schrenk co-authored a presentation at the V.M, Goldschmidt Conference in Prague in August, 2011 with J. Amend (USC) and D. Meyer-Dombard (UIC) comparing the microbial biogeography of terrestrial, shallow-sea, and deep-sea hydrothermal vent ecosystems. This work is currently being prepared for publication.
5.1.2 Novel Culturing Approaches
The Schrenk’s Lab continues to develop and apply novel methods of cultivation to study the microbiology of extreme ecosystems, and as a point-of-comparison to more traditional methods. One line of inquiry involves the use of in situ colonization experiments containing mineral substrata native to the natural environments (e.g. olivine). Experiments have been deployed and recovered from the Tablelands Ophiolite complex, and are currently being developed for the subsurface boreholes at the CRO. These experiments are being used to evaluate succession of colonization kinetics of microbial communities as they relate to changes in mineralogy (e.g. serpentinization or mineral carbonation). Through careful analysis of the microbe-mineral interactions, they hope to gain insight into the linkages between serpentinization, fluids, and life. Second, they have employed stable isotope tracer experiments consistently across all of our field sites to follow the conversion and assimilation of 13-C labeled organic compounds (methane, carbon dioxide, acetate, formate) related to microbial activities. Thirdly, they are using conducting gas consumption-production experiments in microcosms using amended seep waters from each of the serpentinites being studied.
These experiments provide clues to developing cultivation strategies and insight into controlling factors upon the natural microbial communities. For example fermentation of small organic molecules and reduction of ferric iron both appear to support the growth of microorganisms from our terrestrial serpentinites, whereas methanogenesis does not (in contrast to the Lost City ecosystem). Results of this work are being presented at the Fall 2011 AGU meeting by Schrenk and Brazelton (invited talks) and Nelson (poster). Furthermore, traditional approaches using organic-rich media at high pH have yielded cultivars from each of the serpentinite ecosystems being studied (Fig. 1), whose growth characteristics are being determined, and which will be used for genomic comparisons.
5.1.3 Structural and Compositional Analysis of Biofilms
The Lost City hydrothermal system is of particular interest to the Baross’ team because it may have been one of the important settings for crucial steps in the origin of life and for the earliest microbial ecosystems. They have previously reported that the carbonate chimneys at the Lost City hydrothermal field are coated in biofilms dominated by a single phylotype of archaea known as Lost City Methanosarcinales (LCMS). Their recent results W. J. Brazelton et al., 2011a show surprising physiological complexity in the single-species biofilms, which is typically indicative of multi-species biofilm communities. Multiple cell morphologies were detected within the biofilms by transmission electron microscopy, and some cells contained intracellular membranes that may facilitate methane oxidation. Both methane production and oxidation were detected at 70-80 °C and pH 9-10 in samples containing the single-species biofilms. Both processes were stimulated by the presence of hydrogen (H2), indicating that methane production and oxidation are part of a syntrophic interaction (Syntrophy is a biological relationship in which microorganisms of two different species or strains are mutually dependent on one another for nutritional requirements). Metagenomic data included a sequence encoding AMP-forming acetyl CoA-synthetase, indicating that acetate may play a role in the methane-cycling syntrophy. A wide diversity of nitrogen fixation genes was also identified, which we interpret to have been acquired via lateral gene transfer (LGT). Our results indicate that cells within these single 'species’ biofilms may have differentiated into multiple physiological roles to form multicellular communities linked by metabolic interactions and LGT. Communities similar to these Lost City biofilms are likely to have existed early in the evolution of life Goldman et al., 2011. One of the implications from these finding is that the multicellular characteristics of ancient hydrogen-fueled biofilm communities could have stimulated ecological diversification as well as unity of biochemistry during the earliest stages of cellular evolution.
The Baross’ team has expanded their studies of the sub-surface environments associated with hydrothermal vents, to include viruses and their possible role in effecting the genetic landscape of the microbial communities. They have obtained a metagenomic of a viral assemblage from 170 liters of diffuse-flow fluids from a hydrothermal vent on the Juan de Fuca Ridge. They will also be getting a metagenome of the bacterial and archaeal community from the same sample. It is well known that viruses can directly influence the genetic capabilities and the fitness of their hosts through the use of fitness factors and through horizontal gene transfer. However, there is very little known about the impact of viruses on deep sub-surface microbial communities. Subsurface habitats connected to hydrothermal vent systems are characterized by constant fluid flux, dynamic environmental variability and high microbial diversity. In such conditions, high adaptability would be an evolutionary asset, and the potential for frequent host-virus interactions would be high, increasing the likelihood that cellular hosts could acquire novel functions. They have obtained evidence supporting this hypothesis, including data indicating that microbial communities in subsurface hydrothermal fluids are exposed to a high rate of viral infection, as well as viral metagenomic data suggesting that the vent viral assemblage is particularly enriched in genes that facilitate horizontal gene transfer and host adaptability Anderson et al., 2011a. Therefore, viruses are likely to play a crucial role in facilitating adaptability to the extreme conditions of these regions of the deep biosphere.
The long-term goals from these studies are to better understand the role of viruses as agents of lateral gene transfer, the genes transferred, and whether or not there is inter-phylum/domain horizontal gene transfer. Some emphasis will be on viruses that infect hyperthermophilic Archaea as a possible model system for understanding the origin and evolution of viruses and their role in the early diversification of life.
Post-doc Brazelton along with Co-PI Baross published a paper on the physiological diversity of microbial biofilms from the walls of carbonate chimneys at the Lost City Field W. J. Brazelton et al., 2011a. These observations are being followed up on by Schrenk’s group in terrestrial serpentinites with small-scale DNA fingerprinting studies, and through the cultivation experiments described above. Additionally, as described earlier, students in Schrenk’s lab continued to refine methodologies to quantify microbial distribution in rock-hosted environmental matrices.
5.1.4 Adaption of Organisms to Cold Environments
J. Vidonish (student intern) and Co-I M.L. Fogel have been working on identification of the origin of organic matter (OM) in Arctic ice, which is of key importance in understanding the nature and origin of life in both modern and ancient ice. In turn, knowledge of this OM is important for studying biosignatures of ice, necessary for the search for life on Mars. In a study of samples from Svalbard, Norway, both stable isotope analysis and the presence of a unique UV-absorbing pigment (scytonemin) serve as a means of tracing potential sources of organic matter to glacial ice. Possible sources for glacial OM include the following: 1) Aeolian sources including black carbon or local soils, creating “dirty snow”; 2) Plant sources from surrounding areas including higher plants, lichens, and mosses; 3) Microbial sources from on the ice itself, i.e., snow algae and cryoconites; and 4) Microbial biofilms and endoliths from nearby habitats.
The UV-sunscreen pigment scytonemin is prevalent in many of the sources, particularly snow algae, cryoconites, lichens and microbial biofilms, as determined by spectrophotometry and ultra-performance LCMS analysis. These same methods also revealed the presence of scytonemin in various ice cores and surface runoff samples, suggesting that these plant and microbial sources are indeed tied to the introduction of OM to the ice of Svalbard. Supporting pigment signature tracing, stable isotope analysis of 13C and 15N shows a clear trend confirming the origin of OM from regional organic matter, as both ice cores and runoff have an isotopic signature comparable to Svalbard plants, snow algae, cryconites, and lichen. These two lines of evidence conclude in tandem that the OM found in Svalbard’s glaciers have an origin in the surrounding ecosystem Vidonish et al., 2011. This finding suggests that traces of life can both be transported and preserved in ice for extended periods of time. This has especial meaning in the pursuit of biosignatures on Mars, as well as the study of paleoclimate on Earth. Future studies can focus on further tracing of biogenic material from source location to ice, both locally and through long-distance wind transportation.
Collaborator V. Starke, with collaborator J. Kirshtein (USGS, Reston, VA) and CO-Is M.L. Fogel and A. Steele has been focusing on a critical question in microbial ecology concerns how variations in environmental conditions affect microbial community makeup. Arctic thermal springs provide an exceptional opportunity to study this question because they have very steep gradients in temperature, moisture, and mobility that place strong selective pressures on microorganisms. Troll Springs (79°23’N, 13°26E) in the Svalbard archipelago, are one of the northernmost documented thermal springs on land. Precipitation of travertine (calcium carbonate) from Troll’s carbonate-rich waters has built a complex terrace structure. Biological materials are present at all levels of the spring structure.
To investigate this microbial community in detail, we analyzed DNA extracted from wet biofilms, granular samples and endoliths with 454 parallel-tagged pyrosequencing and automated ribosomal intergenic spacer analysis (ARISA) Starke et al., 2011a. The aim is to provide a comprehensive overview of how the community at Troll Springs changes over the gradients in environmental conditions present, such as warm to cold. The 454 and ARISA data were analyzed using multivariate methods, including non-metric multidimensional scaling (nMDS). Results show a gradual transition in the makeup of the microbial community as the environment changes from aquatic to lithologic. These observations suggest a mechanism by which the rocks are colonized by microorganisms: biofilm becomes entrapped during carbonate precipitation.
Use of a range of parameters and techniques in the data processing and multidimensional scaling provides additional insight into how community makeup varies across the environments present at the spring. Some more adaptable species are found across most environments, but change markedly in abundance as the conditions change. Other less adaptable species are found in fewer environments, while being entirely absent in most of the environments. Continued analysis will help reveal which species are the most adaptable, and how their adaptive capabilities permit them to colonize an extreme environment like an arctic endolith Starke et al., 2011b.
This study will provide novel information about microbial adaptation mechanisms and community structure under changing environmental conditions. Establishment of diversity patterns provides an overview of changes in the communities over the gradients in the system. Similar environmental gradients may be important in other settings of astrobiological significance.
5.1.5 Microbial Diversity – Gypsiferous Desert Environments
Collaborator M. Glamoclija and Co-Is A. Steele and M.L. Fogel have been investigating gypsiferrous desert environments at White Sands National Monument from New Mexico. Sulfate-rich salts have been identified as important and widespread component of Mars sedimentary deposits. The presence of Noachian/early Hesperian sulfate-rich deposits have been identified by the Mars Exploration Rover Opportunity at Meridiani Planum and by Mars Reconnaissance Orbiter in sedimentary sequences within Gale crater, the Mars Science Laboratory landing site. The detected sulfate rich beds have been inferred as formed in dune field and more possibly in playa setting. The study of terrestrial analogue site such as White Sands National Monument from New Mexico, provides an analogue system to explore and characterize the proposed habitable zones and their potential biosignatures.
Study of the gypsum dune field partially overlaps with last year’s report and these data are published in Glamoclija et al., 2011a. Deposits from dune sides and interdune areas were investigated to determine the characteristics of microbial habitat and communities through mineral assemblages, microbial pigments along with investigations of nitrogen and sulfur cycles. This year we have focused on mineral analyses and found that microbes with metabolic abilities for sulfur cycling (i.e. dissimilatory sulfite reducers, purple sulfur bacteria, green sulfur and non-sulfur bacteria, and organisms with the APS enzyme) that were identified in all samples were relevant for formation of trace mineral precipitates Glamoclija et al., 2011b; Glamoclija et al., 2011a. These particular organisms have the ability to reduce sulfate and to re-oxidize reduced sulfur compounds back to sulfate. Some of the minor and trace mineral phases associated with biofilm (e.g. a variety of carbonate minerals and celestine) have precipitated due to microbial presence and/or activity. The EDS data showed that fresh microbial biofilm was enriched in Cl, Mg, Ca, Al and Si compounds. The cyanobacterial biofilm may initially inhibit carbonate precipitation by accumulating free Ca2+ in the organic matrix. Degradation/recycling of the biofilm by sulfate reducing organisms (e.g. DSR) and other heterotrophs results in release of elevated Ca2+, which locally favors precipitation. Additionally, cyanobacteria can increase alkalinity during photosynthesis and sulfate reducers may locally alter pH by sulfate consumption, both of which would favor carbonate precipitation. Microbial sulfate reduction has been suggested as a possible mechanism for celestine precipitation. The results characterizing microbe-mineral interactions have been presented at 42nd LPSC conference in March 2011, and the paper summarizing our results from the dune environments is in press in Geomicrobiology Journal.
A deflationary basin, Alkali Flat, holds active playas and a playa lake, which allows for an excellent comparison of these different (hyper)saline habitats Glamoclija et al., 2011c. Additionally, dome structures in the area contain coarse selenite crystals, an interesting alternative microbial habitat. Comparison of environmental physicochemical conditions and molecular biology was used to determine the characteristics of microbial habitats and communities. Predominance of nitrates over ammonium in most of the settings implies that nitrification processes might be important in these ecosystems. Reverse trend was detected only in bottom deposits of playa lake indicating reducing chemistry. Ammonium oxidizing organisms were detected in all samples, thereby indicating microbial involvement in nitrification. Further, nitrogen fixing, denitrifying organisms and anammox bacteria were present in most of the samples. Microbes with metabolic abilities for sulfur cycling (i.e. dissimilatory sulfite reducers and anoxyphototrophs) were identified in all samples. Carbonate mineral phases associated with biofilm in selenites and lake deposits were likely related to microbial sulfate reduction. Whereas potassium chlorite phase detected with biofilm in playa sediments likely resulted from stunted microbial sulfate reduction, therefore indicating microbial response to multiple environmental stresses. These data will be presented at the AGU conference in December 2011, and the paper is in preparation for Geomicrobiology Journal.
5.2 Microbial Adaptations
High pressure resistance in Prokaryotes was studided by CIW group that consisted of P. Griffin (research assistant), A. Kish (former CIW postdoctoral associate, at present postdoctoral associate at Paris Sud University), Co-Is M.L. Fogel, A. Steele and R.J. Hemley
Co-I M. Schrenk and collaborators B. Brazelton, B. Nelson, and H. Blumenfeld are conducting several lines of research with regards to linking abiotic carbon chemistry to microbial metabolism and growth.
5.2.1 Adaptation of Halophiles to High Pressure
P. Griffin, A. Kish, M.L. Fogel, A. Steele and R.J. Hemley, from the CIW, examined the high-pressure survival of a range of prokaryotes not found in high-pressure environments to determine the effects of adaptations to osmotic and oxidative stresses on piezo-resistance. This is continuum study and during the last year results were summarized and a manuscript was submitted to Extremophiles journal. The pressure survivals of Halobacterium salinarum NRC-1, Deinococcus radiodurans R1, and Chromohalobacter salexigens were compared to that Escherichia coli MG1655. C. salexigens, which uses the compatible solute ectoine as an osmolyte, was as piezo-sensitive as E. coli MG1655, suggesting that ectoine is not a peizolyte. D. radiodurans R1 and H. salinarum NRC-1, both resistant to oxidative stress, were found to be highly peizo-resistant. H. salinarum NRC-1 showed nearly full survival after pressurization up to 400 MPa; a survival 3.5 log units higher than E. coli MG1655. This peizo-resistance was maintained for pressurizations up to 1 hr. The protein profile of H. salinarum NRC-1 showed regulated response after pressurization at 300 MPa for 30 min, indicating protein stability and an ability to regulate translation absent from E. coli MG1655 under the same conditions. We propose that the resistance of H. salinarum NRC-1 is due to a combination of factors including cell envelope structure and the presence of intracellular salts, which are hypothesized to stabilize proteins under high-pressure conditions.
5.2.2 Linking Abiotic Carbon Chemistry to Microbial Respiration
Co-I M. Schrenk and collaborators B. Brazelton, B. Nelson, and H. Blumenfeld are conducting several lines of research with regards to linking abiotic carbon chemistry to microbial metabolism and growth. In serpentinite ecosystems, stable isotope probing and cultivation experiments are being used to follow microbial growth and activities relative to certain carbon and energy sources (Fig. 2). Complementary to this work, genomics methodologies are being applied to samples obtained from the natural environments. Brazelton, Nelson, and Schrenk recently published a paper in Frontiers in Extreme Microbiology highlighting insights into energy metabolism and carbon cycling gained through the comparative metagenomic analyses of serpentinite ecosystems. Furthermore, we continue make efforts to “nest” our microbiological analyses within a detailed and consistent geochemical framework across all of our study sites. These involve careful coordination with an international team of collaborators with expertise in isotope geochemistry, organic geochemistry, mineralogy, etc.
Similarly work by ECU Master’s student H. Blumenfeld provided data related to the phylogeny and biogeography of hydrothermal vent chimney communities harboring genes for carbon fixation. Her analyses comprised quantitative PCR assays as well as classic cloning and sequencing methodology. These data are being integrated with a suite of chemical data, including stable isotopic analyses of carbon and nitrogen to link microbial activities to energy and nutrient availability in the chimney environment.
5.3 Biosignatures
J. Farquhar group includes: Aubrey Zerkle (a former post doctoral researcher – now a lecturer at Newcastle University), Harry Oduro (a former graduate student – now a postdoctoral researcher at MIT), Andrew Masterson (a former undergraduate student – now a graduate student at Harvard University), Brian Harms (current graduate student), Daniel Eldridge (current graduate student), Jonathan Banker (current undergraduate student). Eight papers resulting from this work were published in 2010-2011, and several ongoing studies are to be completed and published in the next year. Farquhar group has been studying sulfur isotope fractionations using single- and multicellular cellular organisms, different sets of modern environments (lake, marsh and hydrothermal systems), early rock record and abiotic photochemical and thermal processes.
D. Papineau (former postdoctoral fellow at CIW, now assistant professor at Boston College) worked in CIW team with E. Hauri, M. Fogel and G. Cody. Papineau investigates sulfur and carbon isotope geochemistry to decipher the earliest rock record (particularly BIFs) and characterize occurrence of potential biosignatures.
The rest of CIW team working on different aspects of biosignatures includes A. Steele, R. Hazen, D. Sverjensky, W. Guo (Postdoc) and R. Bowden (lab manager, Fogel group), M. Glamoclija (research scientist), D. Bower (postdoc), J. Armstrong (microbeam specialist), D. Hummer (postdoc), P. Griffin (research assistant), D. Smith (graduate student). Besides sulfur and carbon isotopes, this group has studied hydrogen isotopes, Raman spectroscopy, bacterial pigments and mineral evolution to characterize and evaluate a range of biosignatures
5.3.1 Sulfur Isotopes
Work during the past year by the J. Farquhar group has focused on four areas of sulfur isotope geochemistry: (1) studying sulfur isotope effects associated with metabolic transformations of sulfur by single cellular and multicellular organisms Canfield et al., 2010; (2) evaluating the expression of these effects in modern lake, marsh, and hydrothermal systems Li et al., 2010; Oduro et al., 2011a; Zerkle et al., 2010; (3) understanding sulfur isotope signals associated with the early rock record (Archean and Proterozoic) Farquhar et al., 2011; Shen et al., 2011; and (4) evaluating sulfur isotope effects associated with photochemical and thermal abiological processes to provide a context for evaluating biological effects in early environments Masterson et al., 2011; Oduro et al., 2011b.
Notable findings for the funded period include:
- The documentation of isotope fractionations of 60->70 ‰ in laboratory incubations of natural populations of a sulfate reducers (related to D. thiozymogenes) and a natural substrate Canfield et al., 2010. Note that similar magnitude fractionations have been recently documented in pure culture in Sub Sim et al. (2011). This was listed in last year’s report as well.
- Use of continuum sulfur cycle models to demonstrate that sulfur disproportionation is sometimes consistent with observations and sometimes not required to explain observations in systems with high sulfur isotope fractionations Li et al., 2010; Zerkle et al., 2010. This was listed in last year’s report as well.
- Development of new protocols for extraction of sulfur and analysis of multiple sulfur isotopes in methylated sulfur compounds that allow for tracking of the pathways by which these compounds are formed Oduro et al., 2011a.
- Studies of sulfur cycling in ancient systems including a review paper Farquhar et al., 2011 and a study that examines an unusual isotope signature at the boundary that coincides with the end Permian mass extinctions and implies sulfur cycling in a nonsteady state system which was suggested to result from shutting off of bioturbation by rising sulfidic deep waters.
- A study that describes how changes in the amount of a transparent bath gas generate changes in the character of photochemical mass-independent isotope effects produced in closed-cell laboratory experiments Masterson et al., 2011. This study strongly suggests a primary photochemical isotope isotope effect in addition to any shielding effects present in the experimental design. It also demonstrates that the relationship between Δ33S and Δ36S will depend on pressure and the efficiency of collisional interactions in photochemical atmospheres.
- A study that strongly suggests the thermal reaction pathways that reduce sulfate during their interaction with amino acids is associated with spin selective interactions involving an ion-radical polymerization mechanism Oduro et al., 2011b. This finding raises the possibility of such effects being present in and useful for study of oil maturation environments, and suggests that thermal reactions are not the source of mass-independent effects seen in the early rock record.
Ongoing work with biological experiments includes analysis of two sample sets from laboratory culture experiments with sulfate reducers. One of these is led by Brian Harms (current graduate student) who is completing a study of A. fulgidus cultured at temperatures from 45 to 90 °C. His results allow him to assign changes in the magnitude of the fractionation between sulfide and feed sulfate to changes in the transport and enzymatic function of this organism over this temperature range. A second study with D. autotrophicum cultures grown using a 17O-labelled sulfate substrate and three different electron donors (Hydrogen, butyrate, and acetate) is being undertaken by Jonathan Banker (current undergraduate student) as part of his senior thesis to explore the metabolic function of this organism under different growth conditions. A study is in preparation by Oduro to describe the metabolic transformations of sulfur associated with the assimilatory pathways and subsequent methelation pathways used by macro and micro algae. Another research focus is related to an effort by Daniel Eldridge (a Ph.D. student) to study the isotope effects associated with abiological oxidation pathways of S(IV) and sulfide in aqueous solutions. Daniel has made progress designing his experiments for oxidation and will focus on these experiments during the next year. They provide a basis for comparison with biological effects examined by Zerkle in prior NAI supported work.
5.3.2 Sulfur Isotopes
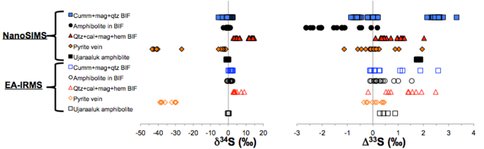
D. Papineau and E. Hauri have developped the analytical method for multiple sulfur isotope analyses with the Cameca NanoSIMS 50L. The development of this method has been continuing effort since 2006 and has now produced several datasets that are being progressively prepared for publicaiton. Using a beamsize of 15×15 microns, we are able to achieve a 2-sigma reproducibility on standards better than +/- 1.0 and +/- 0.3 permil for δ34S and Δ33S values, respectively. Their NanoSIMS survey of sulfides from >3.8 Ga Nuvvuagittuq Supracrustal Belt (NSB) BIFs and associated rocks has revealed a range of about 20 permil in δ34S (between +14 and -5 permil – excluding veined sulfides, see Fig. 3) and positive and negative Δ33S values between -2.6 and +3.4 permil (Fig. 3). To independently verify these analyses they have drilled these sulfides and analyzed them by isotope ratio mass spectrometry in continuous flow (CF-IRMS), in Papineau’s Precambrian Biogeochemistry and Exobiology Laboratory (PBEL) at Boston College. This yielded similar ranges of delta-values, but some differences were noted where some sulfides from amphibolites had positive Δ33S values when analyzing these larger domain aliquots. These differences may be explained by possibility that micron-size sulfide subdomains preserve larger isotope range probably because we are preserving small scale fractionation effects that could have occurred in a closed system. In such systems, Rayleigh-type distillation processes might have created small heterogeneous subdomains that are averaged when looking at bulk methods like CF-IRMS, such as the analyses of drilled aliquots of hundreds of micrograms of sulfides. These results were presented at the recent 2011 Goldschmidt Conference in Prague, Czech Republic.
Multiple Sulfur Isotopes: M.L. Fogel, Weifu Guo (Postdoc) and Roxane Bowden (Lab Manager) have been focusing on multiple sulfur isotope analyses of geological materials, as they play important roles in our understanding of the environmental and climate conditions on Earth at present and in the past. In September 2010, Fogel and colleagues submitted a proposal to the NASA Astrobiology Director’s Fund to purchase a new elemental analyzer (EA) and to develop a continuous flow mass spectrometric method for simultaneous determinations of the C, N and multiple S isotopic compositions (i.e., δ13C, δ14N, δ34S, Δ33S) of organic materials, by coupling the vario MICRO cube elemental analyzer (Elementar Americas, Inc.) with the W. M. Keck-funded Finnigan Delta-V mass spectrometer, especially designed for S analysis of ancient rocks and materials. Once developed, this method will be a rapid, one-step procedure for the analysis of multiple sulfur isotopes and will greatly improve our understanding of the carbon, nitrogen and sulfur biogeochemical cycles on the Earth at present and in the past, and of S isotope signatures in meteoritic organic matter and other astrobiological materials.
This work builds upon our very recent developments of the multiple sulfur isotope analysis of SO2 gas [Guo et al. 2010], and will enable simultaneous determination of C, N and multiple S isotopic compositions of organic samples and thus the tracing the fate of these materials in geological cycles. The C, N and S isotopic studies of different biogeochemical, meteoritic, and experimentally generated materials are the focus of several NASA Astrobiology teams (e.g., CIW, PSU, Goddard, MIT). This proposed work is expected to greatly expand our understanding the interactions among carbon, nitrogen and sulfur geochemical cycles on our planet Earth and the organic chemistry in planetary systems. Currently, we are working on the analytical methodology to perfect the technique for routine samples.
5.3.3 Carbonaceous Biosignatures
D. Papineau, M.L. Fogel and G. Cody have been performing detailed petrographic surveys of apatite grains in association with carbonaceous material (CM) in several Paleoproterozoic, Neoarchean and Eoarchean banded iron formations (BIFs). Petrographic and Raman spectroscopic surveys of Paleoproterozoic BIFs show that apatite grains typically occur as bands parallel to bedding and are often associated with CM that likely represents diagenetically alterated biomass. Combined with petrographic and crystallographic observations, the isotopic and elemental compositions of the Akilia graphite provide additional information to constrain the possible origin(s) of graphite associated with apatite in the Akilia Qp rock. Targets extracted by the focused ion beam (FIB) method contained thin graphite coatings on apatite grains rather than inclusions sensu stricto as inferred from transmitted light microscopy and Raman spectroscopy. While several observations point to graphitization from hydrothermal fluid-deposition for graphite in Eoarchean BIFs, sources of carbon for the inferred CO2 and CH4 fluids may have included both mantle and decayed biological CM. Graphitization of carbon directly from biogenic CM is unlikely in the Akilia Qp rock. However, while biogenic sources of carbon in the Akilia Qp rock cannot be excluded, abiogenic sources of carbon cannot be ruled out either, and therefore there remain ambiguities for the origin of carbon in this ancient metasedimentary rock. These ambiguities will hopefully be solved eventually through similar systematic and thorough studies of other apatite-graphite associations in a wider range of Eoarchean BIFs.
The same methods were applied to BIFs samples from the Eoarchean Nuvvuagittuq Supracrustal Belt (NSB) in northern Québec. Here, Raman analyses revealed that the CM has strong G- and D-band peaks (at ~1575 cm-1 and ~1345 cm-1, respectively) that point poorly crystalline graphite (PCG) that likely crystallized at low temperature (<400°C). Two of these apatite-associated PCG particles were extracted with the FIB method and analyzed by transmission electron microscopy (TEM) and synchrotron-based scanning transmission X-ray microscopy (STXM). These analyses yielded images of sub-micron minerals associated with PCG that formed under the influence of low-temperature hydrothermal fluids, which together with the Raman data point to a source of carbon that is younger than the rocks – that is, that the PCG did not undergo the same metamorphic history as the rock. These results collectively indicate that apatite+graphite can be fluid-deposited and may not represent a biosignature a robust as previously thought. Current work is focused on our recent discovery of apatite+carbonaceous material in a martian meteorite.
Another research avenue that we are currently pursuing is the oxygen isotope compositions of apatite determined in situ by SIMS. This work was initiated through collaboration between NAI at CIW and the NAI at University of Hawai’i at Manoa, with Dr, Gary Huss. The use the Cameca ims 1280 SIMS to measure d18O values of apatite crystals in BIF and in optically homogeneous apatite crystals has yielded a small unexplained range of deviation from predicted values (determined by TCEA-IRMS), which we are currently investigating using electron backscattered diffraction, as we are suspecting that there may be a crystallographic orientation effect in the SIMS that is not corrected in our treatment of the data. These data are too preliminary to present at this time.
5.3.4 Carbon Isotopes of Paleoproterozoic Rocks and Meteorites
The accumulation of atmospheric oxygen in the Paleoproterozoic atmosphere occurred over several hundred millions of years. Carbon isotope excursions in Paleoproterozoic interglacial carbonates from the Transvaal Supergroup in South Africa and in post-glacial carbonates on most continents indicate a relative increase in burial rates of organic carbon and suggest a significant production of atmospheric oxygen. Because atmospheric oxygen is primarily produced by oxygenic photosynthesis, these observations lead to the suggestion that the cause of atmospheric oxygenation may have been high productivity by cyanobacteria due to the concomitant increased delivery of riverine phosphate. This hypothesis is investigated in the Lower Aravalli Group in Rajasthan.
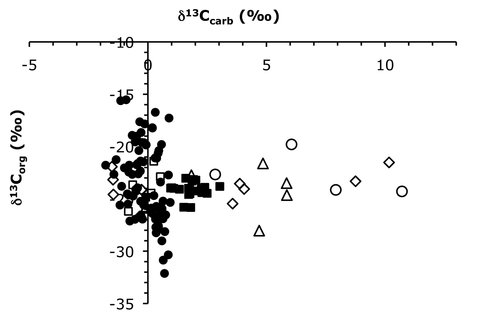
D. Papineau and M.L. Fogel are especially interested in the connection between the occurrence of phosphate deposits and δ13Ccarb excursion, since this connection is likely to indicate a significant cause-and-effect for-and-of high primary productivity at that time as a result of high nutrient availability. New carbon isotope data collected from dolomite from four distinct basinal types in the Jhamarkotra Fm., in India, preserve different geochemical characteristics according to their occurrences in the phosphate domain (PD) or non-phosphate domain (NPD). Dolomite from the NPD often has high δ13Ccarb values, whereas stromatolitic phosphorites from the PD often have high δ 13Corg values and near-zero δ13Ccarb values, which is shown in Fig. 4. The chemical composition (H/C and O/C ratios), Raman spectral characteristics, and synchrotron-based STXM spectra of organic matter extracted from a large selection of these various samples indicate some degree of graphitization, but insignificant alteration of geochemical compositions by metamorphism. Collectively, carbon isotope data for organic matter and carbonate throughout the Jhamarkotra Fm. show different geochemical signatures for high levels of primary productivity that are intimately related depositional environments. D. Papineau and M.L. Fogel suggest that high weathering rates and the consequent increased delivery of phosphate to seawater along this Paleoproterozoic rifted margins stimulated blooms of oxygenic photosynthesis. This scenario may have been a widespread phenomenon during the Paleoproterozoic and may ultimately have been responsible for atmospheric oxygenation at that time.
Current work is expanding in the study of carbonates deposited immediately after the GOE, which is a poorly documented time-period, to see if perturbations in the carbon cycle continued, albeit attenuated, after the GOE. For this task, a sample collection expedition to the Belcher Islands, Nastapoka Arc, and Richmond Gulf was performed from 15-31 July 2011, and was funded by the NASA Astrobiology Institute, the Carnegie Institution of Canada, the NASA Exobiology and Evolutionary Biology Program, and the Keck Foundation. The expedition took place on a ship and was lead by Papineau and included participants from Boston College, the Carnegie Institution of Washington, the Massachusetts Institute of Technology, and the Geological Survey of Canada. Our expertise included structural geology, micropaleontology, biosignatures, organic biomarkers, isotope biogeochemistry, and sedimentary petrology. Over 500 kg of rock samples were collected, and the analysis of these will be the focus of the next several months/years. This work involves an on-going collaboration of CIW-NAI and MIT-NAI on the study of organic biomarkers in the Paleoproterozoic stromatolites from the Belcher Islands.
The primary goal of this study is to establish a comprehensive chemostratigraphy of the Belcher, Nastapoka, and Richmond Gulf groups. Within this goal there are three separate questions: 1) How does the carbonate carbon cycle change throughout the sequence, 2) how does organic carbon cycle change, and how can we add to the knowledge of the carbon excursion that has been gathered thus far, and 3) what do these changes allow us to extrapolate about the alteration of the redox state of the hydrosphere and atmosphere after the GOE.
Roxane Bowden, M. Glamoclija, A. Steele, and M.L. Fogel have been investigating carbon isotope studies on low carbon content materials. It is generally accepted that at least 10 μg C. The δ13C of Peru Mud was -19.92±0.32 ‰ (n=40) for samples in this size range. All samples were weighed using a CP2P Sartorius Microbalance. Weigg of C is required to obtain a reliable carbon isotopic measurement (δ13C), and most labs will quote a range of 20 to 100 μg C. The δ13C of Peru Mud was -19.92±0.32 ‰ (n=40) for samples in this size range. All samples were weighed using a CP2P Sartorius Microbalance. Weigg of C. The need to obtain reliable δ13C values with less than 10 μg of C has become more pressing as the search for evidence of life on Mars increases. The Mars Science Laboratory (MSL) rover, named Curiosity, is set to launch in Fall 2011 with a mission to determine if Mars has ever been habitable. In situ stable isotopic analyses of Martian samples will encompass all carbon-containing phases, including inorganic carbon. The ability to detect and analyze very small amounts of organic carbon in samples will become even more important with the Martian sample return mission. The current evaluation of low organic carbon content was undertaken with a suite of well-known Martian meteorites.
The chemical (ppm C) and isotopic composition (δ13C) of carbon in meteorite samples was determined by combustion in an elemental analyzer (Carlo Erba NC 2500) interfaced through a ConfloIII to a Delta V Plus isotope ratio mass spectrometer (ThermoFisher). Carbon concentrations were calculated using a calibration derived from measuring a homogenous sediment sample (Peru Mud, Penn State Univ.) containing 6.67% C by weight. For this study, 10-100 μg of this standard was weighed out, which corresponds to 0.6 to 6 μg C. The δ13C of Peru Mud was -19.92±0.32 ‰ (n=40) for samples in this size range. All samples were weighed using a CP2P Sartorius Microbalance. Weighing utensils were washed in distilled/Nanopure water and dried at 80 °C. Rock powders were weighed into 4×6mm Ag capsules that were pre-combusted at 599 °C in air for 2 to 4 h to remove organic carbon contamination.
Carbon blanks from capsules were 0.2±0.07 μg (n=72). Measurements are presented without blank correction for isotopic composition. This procedure may fly in the face of convention, however because blanks were below normal detection limits with the Carlo Erba-Delta V system, it was not possible to accurately determine the δ13C of the blank.
A natural, terrestrial, surface-collected basalt sample (Svalbard, Norway) was used as a protocol control. All the samples were processed in glassware baked at 500°C for 8 h. Glass vials and plastic caps with a Teflon lining were used to store the samples. Caps were cleaned by soaking in 10% HCl, followed by a 1 or 2 min sonication, then rinsed with Nanopure water and dried at 40 °C. This basalt, untreated, contained 160 ppm C with a δ13C of -21.3 ±1‰. Approximately 40 mg of powdered basalt was then heated at 599 °C for 2 h to remove surface contamination; this material contained 60 ppm C with a δ13C of -9.7±3.8‰. The enrichment in 13C reflects the removal of terrestrial surface carbon (δ13C typically -26‰) and the presence of residual carbonate and high-temperature macromolecular carbon (MMC). The remainder was treated with a 10% HCl solution for 2 h to remove carbonate minerals. HCl was removed from the samples by rinsing to neutrality with Nanopure water. Acidified samples were then combusted at 599 °C for an additional 2 h, then analyzed. Residual carbon had a δ13C of -24.8 ±6 (n=10) with a concentration of 30 ppm. Typically we weighed out about 10 mg, which contained about 0.34±0.2 μg C. By analyzing the rock samples sequentially, we were able to more easily trace the various pools of carbon: 1) contamination on the surface; 2) carbonate carbon; 3) high-temperature graphitic carbon.
This same protocol was followed for each meteorite sample presented in this study. In general, 10-20 mg of powdered meteorite matrix or separated olivine were weighed for concentration and isotopic measurements. The carboneaceous chondrite, Allende, was used as a positive control sample. The pre-combusted and acidified meteorite contained 186 ppm C with a δ13C of -9.4±0.4 (n=9) reflecting the presence of macromolecular carbon.
Treated Martian meteorites contained between 16-35 ppm C with signal: noise ratios >3, based on peak area, for three different Martian meteorites. Isotopic compositions for these meteorites had standard deviations of 0.5 to 2‰. Although these errors are an order of magnitude greater than typical continuous flow-carbon isotopic measurements, we were able to favorably compare our data with previously published δ13C from similar Martian samples. A fourth meteorite sample had signal:noise ratios of 2.21 and should be treated with greater caution.
How low can you go with conventional continuous flow carbon isotope measurements? With the increasing popularity of Nano-SIMS and other ion beam instruments for measuring carbon isotopes in solid samples, the EA retains a critical role in ground-truthing carbon concentrations and δ13C values for extraterrestrial samples and ancient rocks.
5.3.5. Carbon-Nitrogen-Sulfur-Hydrogen Isotopes
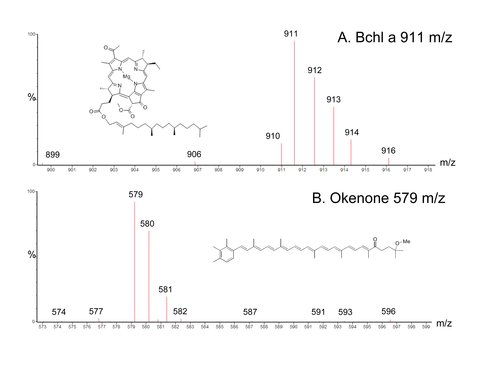
D. Smith, A. Steele, M.L. Fogel and G. Cody have initiated a set of experiments to investigate the biosynthesis of bacterial pigments including purple sulfur bacteria-specific, okenone. Phototrophic sulfur bacteria play an integral part in the anaerobic cycling of sulfur and carbon. The only known fossil of purple sulfur bacteria (PSB) in the geologic record is okenane, believed to be of biologic origin originating from the carotenoid pigment okenone, which has only been documented in eleven extant species of Chromatiaceae. Organic geochemical studies have identified okenane in preserved organic matter in rocks and ancient sediments and further, okenone production has been observed in modern water columns and sediment surfaces. Bacteriochloroyphll a (Bchl a) is a well-studied photosynthetic compound required for photosynthesis in the organisms that possess it, and is used here as a comparative pigment for okenone. We have undertaken a comprehensive study on the biosynthesis of bacterial pigments including okenone and C, N, and S isotopic fractionation during various growth modes in controlled laboratory experiments of purple sulfur bacteria. Cultures of Marichromatium purpuratum 1591, M. purpuratum 1711, Thiocapsa marina 5653, and FGL21 (isolated from the chemocline of Fayetteville Green Lake, NY) were grown under autotrophic and photoheterotrophic (e.g. acetate or pyruvate) conditions in batch cultures. Concentrations of okenone and Bchl a were quantified as a function of time and growth by Ultra Performance-Liquid Chromatography-Mass Spectrometry-Mass Spectrometry (UP-LC-MS-MS) and spectrophotometry. Isotope Ratio Mass Spectrometry (IRMS) was performed on bulk cells and compound specific analysis of Bchl a and okenone to better understand the fractionation associated with the production of the compounds (Fig. 5). Overall okenone and Bchl a concentrations reached μM levels in the cultures, and were dependent upon cell density and growth conditions.
P. Griffin, M.L. Fogel and A. Steele have investigated hydrogen (H) isotopes aspects as sensitive tracers of biochemical processes that can be exploited to answer critical questions in biogeochemistry, ecology, and microbiology. Despite this apparent utility, relatively little is known about the specific mechanisms of H isotope fractionation involved in biosynthesis. In order to understand how organisms incorporate hydrogen from their chemical milieu into biomass, we have cultured the model bacterium E. coli MG1655 in a variety of media composed of deuterium-labeled nutrients and waters. Isotopic analysis of bulk cell mass reveals that the H fractionation between media water and cell material varies as a function of the nutrient source, with commonly used organic food sources (glucose and tryptone) leading to far smaller fractionation signals than non-standard ones (such as formamide, adenine, and urea). In addition, we have completed compound specific isotope analysis of amino acids using combined GC-IRMS. Amino acids harvested from E. coli cultured on glucose in water of varied D/H composition posses an extraordinary range of isotopic compositions (400-600 ‰). Further, these amino acids follow a systematic distribution of D/H where proline is always heaviest and glycine is always lightest. However, when the short-chain peptide tryptone is used in place of glucose, only the non-essential amino acids reflect media water D/H values, suggesting the direct incorporation of some media-borne amino acids into cellular protein. These observations provide a foundation for understanding the cellular routing of hydrogen obtained from food and water sources and indicate that D/H analysis can serve as a powerful probe of biological function.
Katherine Jin (summer intern) working with Cody, Fogel, Griffin and Steele investigated deuterium uptake during exponential growth of E. coli from 10 % D-glucose and culture media containing 10 % D2O. Two methods were employed, first a combination of 1H, 2H, and 13C solid state NMR was employed to determine the magnitude of uptake and any fractionation associated depending on source. This is the first such study to employ solid state NMR. Secondly, Katherine performed tetramethylammonium hydroxide chemopyrolysis and gas chromatography-mass spectrometry to analyze deuterium distributions in bacterial lipids. Katherine Jin wrote up this work and submitted it to the Siemens Foundation Science competition. As of this moment she is a semi-finalist. A talk covering this research will be presented that the American Geophysical Union annual meeting 2011.
5.3.6 Raman and Soft X-ray microanalysis of Microfossils
Dina Bower (postdoctoral associate at CIW), Daniel R. Hummer (postdoctoral fellow at CIW), Co-I Andrew Steele, and Atsushi Kyono, Division of Earth Evolution Sciences, University of Tsukuba, Japan and J. Armstrong (CIW microbeam specialist) have been conducting experimental research to investigate co-evolution of Fe-,Ti-oxides and microbial fossilization during early diagenesis in sandy sediments and characterization of potential biosignatures. This project involves the culturing and growth of mat building cyanobacteria on ilmenite-enriched quartz sands under simulated early diagenetic conditions. The analytical methods include high-resolution microscopic imaging, X-ray diffraction (XRD), scanning electron microscopy (SEM), and micro Raman spectroscopy. The main goal is to understand the role that microbes play in mineral phase changes during early diagenesis to establish mineralogic biosignatures for life detection. Preliminary results show that microbes do passively influence the precipitation of minerals under early diagenetic conditions. The results were presented at the Geobiology Gordon Research Conference (January 2011)3, Goldschmidt (August 2011)5, and the annual Geological Society of America (October 2011)6. The final results will be presented at the December 2011 American Geophysical Union meeting. A manuscript is currently being prepared for submission to the Journal of Sedimentary Research.
D. Bower and A. Steele have been collaborating with Marc D. Fries (Planetary Science Institute, Tucson, AZ) and Lucas Kater (WiTec Ulm, Ulm, Germany) on investigations of carbonaceous material in geologic samples using micro Raman spectroscopic technique. They are investigating use of G- and D-band parameters as a tool for characterization of microbial fossils and life detection. The investigations involve analysis of spectral features from known biogenic and abiogenic carbonaceous materials to constrain the use of G-and D-band carbon characteristics in elucidating the origins and thermal history of carbon in ancient rocks using the non-destructive analytical method micro Raman spectroscopy. A suite of microfossils in sedimentary rock samples of various Earth ages was compared with a set of well-characterized abiologic carbonaceous chondrite samples. The data show correlations between precursor carbonaceous materials and D-band spectral features along with a trend of increasing G-band peak position with increasing thermal maturity. The results of this work complement a growing database for Raman spectroscopic biosignatures and can be used for laboratory-based studies or in situ life detection on Earth or other planets. The results were presented at the Conference on micro Raman and Luminescence in Earth and Space Sciences II (May 2011). A manuscript is currently being submitted to Earth and Planetary Science Letters.
G. Cody and NAI-ORAU post Doc Neal Gupta finished up their study of organic matter preservation in ancient fossils. Working in collaboration with Derek Briggs (Yale), Roger Summons (MIT-NAI PI), Roy Plotnick and Fabien Kenig (University of Illinois, Chicago), David Kilcoyne (Advanced Light Source) and Andrew Scott (Royal Holloway University of London), Cody and Gupta acquired high spatial resolution X-ray Absorption Near Edge Structure (XANES) data on the C-, N-, and O- k edge (i.e. through excitation of the 1s electrons). It was shown that contrary to previous thinking, considerable relic chitin protein complex is preserved in paleozoic arthropod fossils and may in fact be essential for the preservation of the organic fossil record. This work was published this year in the journal, Geology. Cody and Gupta have continued these studies focusing on some of the oldest trilobite samples. We find that relic protein-chitin complex is also very abundant in a 505 Ma trilobite sample from the Wheeler formation; a manuscript detailing this work is in preparation. This work was presented as part of an invited talk at the V. M. Goldschmidt meeting.
5.3.7 Mineral Evolution as a Biosignature
Mineral evolution is based on the premise that the geo- and biospheres have coevolved through a sequence of deterministic and stochastic events. Consequently, the near-surface mineralogy of a planet or moon reflects biological processes Hazen, 2011; Hazen and Papineau, 2011.
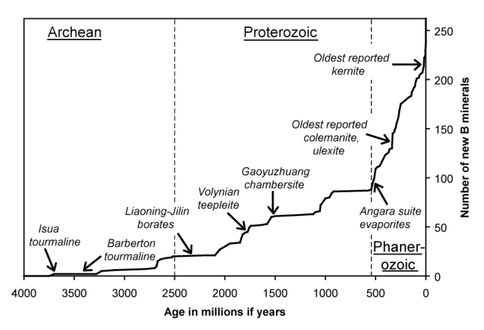
Recent studies have focused on both redox sensitive elements (U, Mo, Hg) and on the minerals of boron. The RNA World is generally thought to have been an important link between purely prebiotic chemistry and modern DNA/protein biochemistry. One concern about the RNA World hypothesis is the geochemical stability of ribose, the sugar moiety of RNA. Prebiotic stabilization of ribose by borate minerals, notably colemanite, ulexite, and kernite, has been proposed as one solution to this difficulty. However, a critical unresolved issue is whether borate minerals existed in significant quantities on the primitive Earth, especially in the period when prebiotic synthesis processes leading to RNA took place E S Grew et al., 2011.
The oldest reported colemanite and ulexite are 330 Ma, and the oldest reported kernite, 19 Ma. However, boron isotope data and geologic context are consistent with an evaporitic borate precursor to 2400-2100 Ma borate deposits in the Liaoning and Jilin Provinces, China, as well as to tourmaline group minerals at 3300-3450 Ma in the Barberton belt (Fig. 6). The oldest boron minerals are tourmalines in the Isua complex (metamorphism at 3700 Ma). Whether borates such as colemanite, ulexite and kernite were present in the Hadean (>4000 Ma) at the critical juncture when prebiotic molecules such as ribose required stabilization depends on whether a granitic continental crust had yet differentiated, because in its absence we see no means for boron to be sufficiently concentrated for borates to be precipitated E. Grew and Hazen, 2010a; E. Grew and Hazen, 2010b.
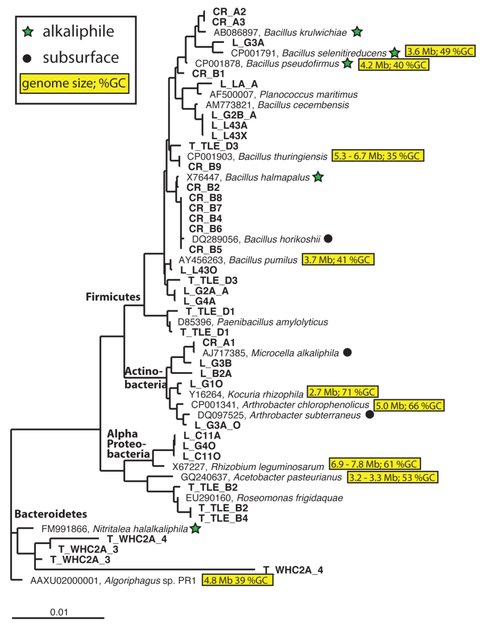
Figure 1. Maximum-likelihood phylogenetic tree (SILVA-aligned, constructed with RaxML in Arb) of 16S rRNA sequences obtained from isolates grown on anoxic, pH 11 media (bold font). Close relatives who are alkaliphilic (green stars) or were isolated from subsurface environments (black circles) are also shown. Close relatives with complete genome sequences are also shown with their genome size and GC%.
Figure 2. A photograph on the left shows collection of the first microbiological sample taken directly from serpentinite subsurface. Photograph on the right shows the serpentinite core.
Figure 3. Comparison of multiple sulfur isotope data by nanoSIMS (filled symbols) and by CF-IRMS of Eoarchean sulfides in BIFs and associated volcanic and metasedimentary rocks from the NSB.
Figure 4. Carbon isotope compositions of organic matter and carbonates from dolomites associated with phosphate (PD – black symbols) and other dolomites from the co-eval open marine environments (NPD – open symbols).
Figure 5. An MS chromatogram showing the successful isolation of okenone and bacteriochlorophyll a.
Figure 6. Cumulative plot for 262 boron minerals based on reported occurrences and age determinations in the literature, showing boron mineral occurrences.
Publications
-
Alexander, C. M. O. D., Newsome, S. D., Fogel, M. L., Nittler, L. R., Busemann, H., & Cody, G. D. (2010). Deuterium enrichments in chondritic macromolecular material—Implications for the origin and evolution of organics, water and asteroids. Geochimica et Cosmochimica Acta, 74(15), 4417–4437. doi:10.1016/j.gca.2010.05.005
-
Anderson, R. E., Brazelton, W. J., & Baross, J. A. (2011). Is the Genetic Landscape of the Deep Subsurface Biosphere Affected by Viruses?. Frontiers in Microbiology, 2. doi:10.3389/fmicb.2011.00219
-
Anderson, R. E., Brazelton, W. J., & Baross, J. A. (2011). Using CRISPRs as a metagenomic tool to identify microbial hosts of a diffuse flow hydrothermal vent viral assemblage. FEMS Microbiology Ecology, 77(1), 120–133. doi:10.1111/j.1574-6941.2011.01090.x
-
Brazelton, W. J., & Baross, J. A. (2010). Metagenomic Comparison of Two Thiomicrospira Lineages Inhabiting Contrasting Deep-Sea Hydrothermal Environments. PLoS ONE, 5(10), e13530. doi:10.1371/journal.pone.0013530
-
Brazelton, W. J., Mehta, M. P., Kelley, D. S., & Baross, J. A. (2011). Physiological Differentiation within a Single-Species Biofilm Fueled by Serpentinization. mBio, 2(4), e00127–11–e00127–11. doi:10.1128/mbio.00127-11
-
Brazelton, W. J., Nelson, B., & Schrenk, M. O. (2012). Metagenomic Evidence for H2 Oxidation and H2 Production by Serpentinite-Hosted Subsurface Microbial Communities. Frontiers in Microbiology, 2. doi:10.3389/fmicb.2011.00268
-
Brazelton, W. J., Sogin, M. L., & Baross, J. A. (2010). Multiple scales of diversification within natural populations of archaea in hydrothermal chimney biofilms. Environmental Microbiology Reports, 2(2), 236–242. doi:10.1111/j.1758-2229.2009.00097.x
-
Canfield, D. E., Farquhar, J., & Zerkle, A. L. (2010). High isotope fractionations during sulfate reduction in a low-sulfate euxinic ocean analog. Geology, 38(5), 415–418. doi:10.1130/g30723.1
-
Cody, G. D., Gupta, N. S., Briggs, D. E. G., Kilcoyne, A. L. D., Summons, R. E., Kenig, F., … Scott, A. C. (2011). Molecular signature of chitin-protein complex in Paleozoic arthropods. Geology, 39(3), 255–258. doi:10.1130/g31648.1
-
Farquhar, J., Zerkle, A. L., & Bekker, A. (2010). Geological constraints on the origin of oxygenic photosynthesis. Photosynthesis Research, 107(1), 11–36. doi:10.1007/s11120-010-9594-0
-
Glamoclija, M., Fogel, M. L., Steele, A., & Kish, A. (2012). Microbial Nitrogen and Sulfur Cycles at the Gypsum Dunes of White Sands National Monument, New Mexico. Geomicrobiology Journal, 29(8), 733–751. doi:10.1080/01490451.2011.608111
-
Goldman, A. D., Baross, J. A., & Samudrala, R. (2012). The Enzymatic and Metabolic Capabilities of Early Life. PLoS ONE, 7(9), e39912. doi:10.1371/journal.pone.0039912
-
Grew, E. S., Bada, J. L., & Hazen, R. M. (2011). Borate Minerals and Origin of the RNA World. Orig Life Evol Biosph, 41(4), 307–316. doi:10.1007/s11084-010-9233-y
-
Griffin, P. L., Kish, A., Steele, A., & Hemley, R. J. (2011). Differential high pressure survival in stationary-phase Escherichia coli MG1655. High Pressure Research, 31(2), 325–333. doi:10.1080/08957959.2010.550891
-
Kaye, J. Z., Sylvan, J. B., Edwards, K. J., & Baross, J. A. (2010). Halomonas and Marinobacter ecotypes from hydrothermal vent, subseafloor and deep-sea environments. FEMS Microbiology Ecology, 75(1), 123–133. doi:10.1111/j.1574-6941.2010.00984.x
-
Kish, A., Griffin, P. L., Rogers, K. L., Fogel, M. L., Hemley, R. J., & Steele, A. (2012). High-pressure tolerance in Halobacterium salinarum NRC-1 and other non-piezophilic prokaryotes. Extremophiles, 16(2), 355–361. doi:10.1007/s00792-011-0418-8
-
Li, X., Gilhooly, W. P., Zerkle, A. L., Lyons, T. W., Farquhar, J., Werne, J. P., … Scranton, M. I. (2010). Stable sulfur isotopes in the water column of the Cariaco Basin. Geochimica et Cosmochimica Acta, 74(23), 6764–6778. doi:10.1016/j.gca.2010.08.020
-
Masterson, A. L., Farquhar, J., & Wing, B. A. (2011). Sulfur mass-independent fractionation patterns in the broadband UV photolysis of sulfur dioxide: Pressure and third body effects. Earth and Planetary Science Letters, 306(3-4), 253–260. doi:10.1016/j.epsl.2011.04.004
-
Oduro, H., Harms, B., Sintim, H. O., Kaufman, A. J., Cody, G., & Farquhar, J. (2011). Evidence of magnetic isotope effects during thermochemical sulfate reduction. Proceedings of the National Academy of Sciences, 108(43), 17635–17638. doi:10.1073/pnas.1108112108
-
Oduro, H., Kamyshny, A., Guo, W., & Farquhar, J. (2011). Multiple sulfur isotope analysis of volatile organic sulfur compounds and their sulfonium precursors in coastal marine environments. Marine Chemistry, 124(1-4), 78–89. doi:10.1016/j.marchem.2010.12.004
-
Papineau, D. (2010). Global Biogeochemical Changes at Both Ends of the Proterozoic: Insights from Phosphorites. Astrobiology, 10(2), 165–181. doi:10.1089/ast.2009.0360
-
Papineau, D. (2010). Mineral Environments on the Earliest Earth. Elements, 6(1), 25–30. doi:10.2113/gselements.6.1.25
-
Papineau, D., De Gregorio, B. T., Cody, G. D., Fries, M. D., Mojzsis, S. J., Steele, A., … Fogel, M. L. (2010). Ancient graphite in the Eoarchean quartz–pyroxene rocks from Akilia in southern West Greenland I: Petrographic and spectroscopic characterization. Geochimica et Cosmochimica Acta, 74(20), 5862–5883. doi:10.1016/j.gca.2010.05.025
-
Papineau, D., De Gregorio, B. T., Cody, G. D., O’Neil, J., Steele, A., Stroud, R. M., & Fogel, M. L. (2011). Young poorly crystalline graphite in the >3.8-Gyr-old Nuvvuagittuq banded iron formation. Nature Geosci, 4(6), 376–379. doi:10.1038/ngeo1155
-
Papineau, D., De Gregorio, B. T., Stroud, R. M., Steele, A., Pecoits, E., Konhauser, K., … Fogel, M. L. (2010). Ancient graphite in the Eoarchean quartz-pyroxene rocks from Akilia in southern West Greenland II: Isotopic and chemical compositions and comparison with Paleoproterozoic banded iron formations. Geochimica et Cosmochimica Acta, 74(20), 5884–5905. doi:10.1016/j.gca.2010.07.002
-
Shen, Y., Farquhar, J., Zhang, H., Masterson, A., Zhang, T., & Wing, B. A. (2011). Multiple S-isotopic evidence for episodic shoaling of anoxic water during Late Permian mass extinction. Nat Comms, 2, 210. doi:10.1038/ncomms1217
-
Summons, R. E., Amend, J. P., Bish, D., Buick, R., Cody, G. D., Des Marais, D. J., … Sumner, D. Y. (2011). Preservation of Martian Organic and Environmental Records: Final Report of the Mars Biosignature Working Group. Astrobiology, 11(2), 157–181. doi:10.1089/ast.2010.0506
-
Walter, M., Baross, J., Coustenis, A., Horner, J., Kress, M., Meech, K., … Woolf, N. (2011). Message from the Executive Council of the Astrobiology Society: The First Year. Astrobiology, 11(1), 75–75. doi:10.1089/ast.2011.1050
-
Zerkle, A. L., Kamyshny, A., Kump, L. R., Farquhar, J., Oduro, H., & Arthur, M. A. (2010). Sulfur cycling in a stratified euxinic lake with moderately high sulfate: Constraints from quadruple S isotopes. Geochimica et Cosmochimica Acta, 74(17), 4953–4970. doi:10.1016/j.gca.2010.06.015
- Amaral-Zettler, L., Artigas, F., Baross, J.A., Bharathi, L., Boetius, A., Chandramohan, D., Herndl, G., Kogure, K., Schouten, S., Stal, L., Neal, P., Patterson, D., Pedrol-Alió, C., Thessen, A., De Leeuw, J. & Sogin, M. (2010). A global census of marine microbes. In: McIntyre, A. (Eds.). Life in the World’s Ocean: Diversity, Distribution, and Abundance. London: Wiley-Blackwell.
- Amend, J., Meyer-Dombard, D. & Schrenk, M. (2011). Microbial Community Structures in Shallow-Sea, Deep-Sea, and Terrestrial Hydrothermal Systems. Goldschmidt. Prague, Czech Republic.
- Anderson, R.E., Brazelton, W.J. & Baross, J.A. (2010a). Using metagenomics and CRISPR analyses to study hydrothermal vent viral communities. ISME Conference. Seattle, WA.
- Anderson, R.E., Brazelton, W.J. & Baross, J.A. (2010b). CRISPRs of hydrothermal vent isolates reveal insights into the co-evolution of thermophilic viruses and their hosts. Viruses of Microbes Conference. Paris, France.
- Anderson, R.E., Brazelton, W.J. & Baross, J.A. (2011c). Do viruses affect the genetic landscape of extreme subseafloor habitats? Origins. Montpellier, France.
- Baross, J.A. & Impey, C.D. (2011). Astrobiology – A new synthesis. In: Impey, C.D. & Lunine, J.L. (Eds.). Frontiers in Astrobiology. Vol. in press. Cambridge University Press.
- Baross, J.A. (2010a). Minimal cells, hydrothermal vent biofilms and the origin of multi-cellular communities. Deep-Sea Biology Workshop. Brest, France.
- Baross, J.A. (2010b). Limits of life, early evolution and the search for habitable planets. American Geological Society. Denver, CO.
- Blumenfeld, H.N., Kelley, D.S., Girguis, P.R. & Schrenk, M.O. (2011). Abundance and Distribution of Diagnostic Carbon Fixation Genes in a Deep-Sea Hydrothermal Gradient Ecosystem. American Geophysical Union Meeting. San Francisco, CA.
- Bower, D.M. & Steele, A. (2011a). Diagenetic experiments to track the co-evolution of Fe-,Ti-oxide phase changes and microbial fossilization: establishing potential mineral biosignatures. Geobiology Gordon Research Conference.
- Bower, D.M., Hummer, D.R., Kyono, A. & Steele, A. (2011d). The co-evolution of Fe- ,Ti-oxides and microbial fossilization during early diagenesis in sandy sediments: establishing potential mineral biosignatures. GSA Annual Meeting. Minneapolis, MN.
- Bower, D.M., Kyono, A. & Steele, A. (2011c). Tracking the evolution of Fe-,Ti-oxide phase changes in ilmenites in microbial fossilization experiments: understanding the role of microbes in diagenesis. Goldschmidt. Prague, Czech Republic.
- Bower, D.M., Steele, A., Fries, M.D. & Kater, L. (2011b). Micro Raman spectroscopic investigations of the nature and provenance of carbonaceous material in microfossil bearing rocks: redefining D and G carbon band parameters for the detection of biosignatures. Conference on micro Raman and Luminescence in Earth and Space Sciences II. Madrid, Spain.
- Brazelton, W.J., Anderson, R.E., Mehta, M.P. & Baross, J.A. (2010c). Lateral gene transfer in hydrothermal vents. ISME Conference. Seattle, WA.
- Brazelton, W.J., Ludwig, K.A., Schrenk, M.O., Kelley, D.S., Sogin, M.L. & Baross, J.A. (2010d). Novel insights into methane cycling, lateral gene transfer, and the rare biosphere within carbonate chimneys of the Lost City Hydrothermal Field. American Geophysical Union Meeting. San Francisco, CA.
- Brazelton, W.J., Nelson, B. & Schrenk, M.O. (2011b). Investigating the Potential for Subsurface Primary Production Fueled by Serpentinization. American Geophysical Union Meeting. San Francisco, CA.
- Glamoclija, M., Fogel, M.L. & Steele, A. (2011c). Microbial Nitrogen and Sulfur Cycles at the Playa and Playa-lake Deposits of White Sands National Monument, New Mexico. AGU Conference.
- Glamoclija, M., Steele, A. & Fogel, M.L. (2011b). Microbial influences on aeolian sulfates; A case study of a dune field at White Sands National Monument, New Mexico. 42nd Lunar and Planetary Science Conference.
- Grew, E. & Hazen, R.M. (2010a). Evolution of the minerals of beryllium, and comparison with boron mineral evolution. Geological Society of America Abstracts with Programs.
- Grew, E. & Hazen, R.M. (2010b). Evolution of boron minerals: Has early species diversity been lost from the geological record? Geological Society of America Abstracts with Programs.
- Hazen, R.M. & Papineau, D. (2011). Mineralogical co-evolution of the geo- and biospheres. In: Knoll, A.H., Konhauser, K.O. & Canfield, D.E. (Eds.). A Geobiology Reader. Vol. in press. Wiley-Blackwell.
- Hazen, R.M. (2011). What’s new in mineral evolution. Italy Symposium publication.
- Morrill, P.L., Szponar, N., Brazelton, W.J., Woodruff, Q., Schrenk, M.O., Bower, D.M. & Steele, A. (2010). Life detection at a Mars analogue site of present-day serpentinization in the Tablelands Ophiolite of Newfoundland. American Geophysical Union Meeting. San Francisco, CA.
- Nelson, B., Chowdhury, S., Brazelton, W.J. & Schrenk, M. (2011). Metabolic and Physiological Characteristics of Novel Cultivars from Serpentinite Seep Fluids. American Geophysical Union Meeting. San Francisco, CA.
- Papineau, D. & Hauri, E. (2011b). Isotopically heavy sulfur in banded iron formations from the Eoarchean Nuvvuagittuq Supracrustal Belt. Mineralogical Magazine, 75: 1594.
- Papineau, D. (2010d). On the origin of carbonaceous material associated with apatite in banded iron formation. Proceedings of the 5th International Archean Symposium. Perth, Western Australia.
- Papineau, D. (2011c). Global biogeochemical changes at both ends of the Proterozoic: Insights from phosphorites. Astrobiology Science Conference.
- Papineau, D., De Gregorio, B.T., Steele, A., Stroud, R.M. & Fogel, M.L. (2010e). Fluid-deposition of graphite with apatite in an Eoarchean banded iron formation from the Nuvvuagittuq Supracrustal Belt, Québec, Canada. Geochimica Et Cosmochimica Acta, A790.
- Papineau, D., Purohit, R. & Fogel, M.L. (2010g). Biogeochemical evolution in newly formed rift basins along the Paleoproterozoic Aravalli passive margin. Astrobiology Science Conference.
- Papineau, D., Purohit, R. & Fogel, M.L. (2011d). The Paleoproterozoic rise of Atmospheric oxygen caused by algal blooms stimulated by high phosphate: the Lower Aravalli Group, India. Proceedings of the GSA meeting.
- Schrenk, M.O., Brazelton, W.J., Woodruff, Q., Szponar, N. & Morrill, P.L. (2010). Mapping Microbial Populations Relative to Sites of Ongoing Serpentinization: Results from the Tablelands Ophiolite Complex, Canada. American Geophysical Union Meeting. San Francisco, CA.
- Schrenk, M.O., Nelson, B.Y. & Brazelton, W.J. (2011). The Serpentinite Subsurface Microbiome. American Geophysical Union Meeting. San Francisco, CA.
- Starke, V., Kirshtein, J., Fogel, M.L. & Steele, A. (2011a). Microbial Community Structure Along an Environmental Gradient in the High Arctic. Keystone Symposia on Microbial Communities as Drivers of Ecosystem Complexity.
- Starke, V., Kirshtein, J., Fogel, M.L. & Steele, A. (2011b). Microbial Ecology and Ecosystem Dynamics at an Arctic Thermal Spring. The ISME Journal, submitted.
- Steele, A., McCubbin, F.M., Fries, M., Glamoclija, M., Kater, L. & Nekvasil, H. (2011). Graphite in an Apollo 17 Impact Melt Breccia. Lunar and Planetary Institute Science Conference Abstracts.
- Szponar, N., Morrill, P.L., Brazelton, W.J., Schrenk, M.O., Bower, D.M. & Steele, A. (2010). Present-day serpentinization in the Tablelands, Gros Morne National Park, Newfoundland: a Mars Analogue Site. American Geophysical Union Meeting. San Francisco, CA.
- Vidonish, J., Bowden, R., Benning, L., Eigenbrode, J. & Fogel, M.L. (2011). Tracing the Origins of Organic Matter in the Ice of Svalbard, Norway. AGU Fall Meeting.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Douglas Rumble
Co-Investigator
Rhonda Stroud
Co-Investigator
Dimitri Sverjensky
Co-Investigator
Don Canfield
Collaborator
Katrina Edwards
Collaborator
Jennifer Eigenbrode
Collaborator
Neal Gupta
Collaborator
Julie Huber
Collaborator
David Kilcoyne
Collaborator
Penny Morrill
Collaborator
Dina Bower
Postdoc
Mihaela Glamoclija
Postdoc
Adrienne Kish
Postdoc
Shohei Ohara
Postdoc
Dominic Papineau
Postdoc
Ying Wang
Postdoc
Harry Oduro
Doctoral Student
William Brazelton
Graduate Student
Verena Starke
Graduate Student
Quinn Woodruff
Graduate Student
Katherine Jin
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.1
Biosignatures to be sought in Solar System materials