2011 Annual Science Report
 Carnegie Institution of Washington
Reporting | SEP 2010 – AUG 2011
Carnegie Institution of Washington
Reporting | SEP 2010 – AUG 2011
Project 3: The Origin, Evolution, and Volatile Inventories of Terrestrial Planets
Project Summary
This research project brings together a large team of scientists with a unified goal understanding the origin and evolution of volatiles (C, H, O, and N) in planetary interiors. It includes a theoretical study of planet formation with focus of addressing the abundance of volatiles in objects that ultimately combine to form the terrestrial planets. The project gains from information being currently revealed through the NASA Messenger mission in orbit around Mercury. The project has an experimental component that focuses on studying volatiles deep in planetary interiors using ultra-high pressure devices and molecular spectroscopy for species interrogation. Finally, it includes a systematic study of the chemistry of mineral inclusions in diamonds, where diamond serves to trap minerals in a natural high pressure container. These studies allow CIW NAI scientists probe the chemistry of Earth’s deep mantle and help reveal how Earth’s plate tectonics may have started.
Project Progress
Project 3.1 Models for Planet Formation and Volatile Inventory of the Planets
CoI Chambers’s progress on these tasks consisted of work in two areas: A new numerical model has been developed for the runaway and oligarchic stages of planetary growth. These stages involve the growth of lunar-to- Earth mass planetary embryos from 1-100 km sized planetesimals. The large number of objects involved requires the use of a statistical approach in which objects are divided into a series of mass bins at different radial locations in a protoplanetary disk. Growth and velocity evolution are tracked simultaneously to accurately determine the timescale for growth and the size distribution of bodies. The next steps will be (i) to combine this code with an N-body integrator to model growth all the way to fully formed planets, and (ii) an additional module to track the composition of planets as they grow and collide, beginning with the water mass fraction.
A separate project consists of a new analysis of recent stellar elemental abundance data that may provide constraints on terrestrial planet formation around other Sun-like stars. The data show that elemental abundances vary from one star to another in ways that are strongly correlated with elemental volatility. The Sun is especially depleted in refractory elements. The deficiency can be rectified by adding roughly 4 Earth masses of material with a mixture of Earth-like and chondrite-like compositions. This suggests the Sun’s composition arose due to rocky material that ended up in the terrestrial planets and was ejected from the asteroid belt. A strong correlation between stellar abundance trends and metallicity also suggests that the efficiency of planetesimal formation increases rapidly with metallicity, providing an important input for models of the later stages of planet formation.
3.2. The Inner Solar System: Constraints from Mercury and Mars (Solomon, Nittler)
Co-I Solomon is the Principal Investigator of the MESSENGER mission to Mercury, and Co-I Nittler is a Participating Scientist on the MESSENGER team. As part of this NAI project, Solomon and Nittler are integrating the information derived from MESSENGER into a better understanding of the processes that led to the formation of the small, embryo-sized inner planets, including Mercury at about 5% of Earth’s mass and Mars at about 10%. That the bulk compositions, volatile abundances, magmatic histories, and magnetic field histories differ so strongly on these two bodies demonstrates the strongly stochastic nature of the planet-building process and probably some dependence on solar distance. Because all of these aspects of planetary evolution affect the spatial extent and temporal duration of zones of habitability at the planetary surface and within the shallow planetary subsurface, an improved understanding of the profound differences in the make-up and evolution of these two similar-size planets holds the promise of illuminating the general nature of planetary habitability on smaller Earth-like planets, including those in other planetary systems. For both Mars and Mercury, recent spacecraft observations make such a comparison particularly timely. The ongoing Mars Odyssey, Mars Express, Mars Exploration Rover, and Mars Reconnaissance Orbiter missions continue to build the spectacular data sets from imaging and geochemical and geophysical remote sensing, and the recently completed Phoenix mission augmented our understanding of water and other volatiles at high Martian latitudes. The MESSENGER mission completed its three flybys of Mercury in 2008 and 2009 and was successfully inserted into orbit about Mercury on 18 March 2011. The broad goal of this task is a comparative evaluation of bulk composition, volatile inventory, magmatic history, and core dynamo history on Mars and Mercury, with a focus on aspects of those processes (water availability and circulation, organic material inventory, internal energy, magnetospheric shielding) most strongly relevant to habitability in space and time.
Initial orbital observations of Mercury have allowed the first direct measurements of Mercury’s surface composition by X-ray and gamma-ray spectrometry. These measurements indicate that the surface of Mercury is depleted in Al and Ca and enriched in Mg relative to the terrestrial and lunar crusts, has relatively low total iron and titanium concentrations (< 4 wt% and < 0.8 wt%, respectively), and is surprisingly rich in the volatile elements S and K. Sulfur contents of several weight percent are a factor of ~10 higher than observed in typical surface rocks on the other terrestrial planets, and the K/Th ratio is ~5000, comparable to that of Mars. These compositional data suggest that Mercury formed from a similar mix of precursor materials to those that formed the other terrestrial planets, but substantially more chemically reduced. Planetary accretion models indicate that although there was radial mixing of planetesimals in the inner solar system, any radial gradients in chemical composition should have been partially preserved in the final compositions of the accreted planets. One possible explanation for the chemically reduced nature of Mercury suggested by Co-I Alexander is that Mercury may have formed from precursors enriched in anhydrous, C-rich solids analogous to cometary dust particles. Under this scenario, the other terrestrial planets would have formed from similar solids but with substantially higher abundances of water ice, resulting in more oxidizing conditions.
The vantage point of orbit has permitted MESSENGER to obtain close-up views of the poles of Mercury for the first time and to obtain targeted high-resolution images of much higher resolution than was possible during the flybys, and both opportunities have yielded important new results. A large contiguous expanse of smooth plains, occupying more than 6% of Mercury’s surface area, covers much of Mercury high northern latitudes. These plains show several morphological indicators of a volcanic origin and buried earlier impact craters and basins to depths in excess of 1 km. Regions adjacent to the northern smooth plains show abundant evidence for emplacement of lavas in a flood-basalt style, with high eruption rates and thermal or mechanical erosion of underlying terrain. The northern smooth plains were approximately contemporaneous with the volcanically emplaced smooth plains that lie within and exterior to the 1500-km-diameter Caloris basin, a result confirming that volcanism was a globally extensive process in the era immediately following the late heavy bombardment of the inner solar system.
High-resolution images of bright deposits within impact craters on Mercury reveal numerous fresh-appearing, irregular, shallow, rimless depressions, or hollows. The hollows range from tens of meters to a few kilometers across, and many have high-reflectance interiors and halos. The bright host rocks are interpreted to have been excavated from depth by the crater-forming process. The most likely formation mechanisms for the hollows involve recent loss of volatiles through some combination of sublimation, space weathering, outgassing, or pyroclastic volcanism. These features support the inference from compositional measurements and from observations of pyroclastic volcanic deposits elsewhere on the planet that Mercury’s interior contains higher abundances of volatile materials than predicted by most scenarios for the planet’s formation.
MESSENGER flyby observations showed that Mercury’s internal magnetic field is dominantly dipolar, has a vector moment closely aligned with the spin axis, and displays no evidence for crustal magnetic anomalies. These results support the inference that Mercury’s magnetic field is the product of a dynamo in the planet’s fluid outer core. Orbital magnetic field observations have demonstrated that Mercury’s magnetic equator is located 484±11 km north of the geographic equator, i.e., the best fitting dipole is offset northward from the center of the planet by about 0.2 planetary radii. This offset leads to a substantial north-south asymmetry in the strength of the surface field and in the surface area at high latitudes with open magnetic field lines along which charged particles may readily gain access to Mercury’s surface. The high axisymmetry and strong equatorial asymmetry of the internal field point to a dynamo with characteristics different from those of Earth and other solar system planets.
Section 3.3: Planetary Volatiles
Physicochemical description of melting and crystallization in the interior of the Earth and terrestrial planets in the presence of volatiles (H2O, CO2, CO, CH4, N2, NH3, noble gases, etc.) is fundamental to our understanding of the formation and evolution of the solid Earth, its oceans, and atmosphere formation and evolution of the solid Earth, its oceans, and atmosphere. This information has been obtained with the aid of Raman, infrared, and NMR as well as mass spectroscopic methods to characterize solution mechanisms and various analytical techniques to determine solubility and solution mechanisms and isotope fractionation between fluid and melt while the samples are at appropriate temperature and pressure and by using quenched samples.
During the last year, a central theme has been the role of silicate- and COH-saturated aqueous solutions and COH-saturated silicate melts. The structural data have been employed to develop structure-based models of melts for the purpose of modeling transport properties of hydrous melts and to characterize, on a structural basis, control of element partitioning between melts and aqueous fluids. New methods for pressure measurements in hydrothermal diamond anvil cell at high pressure and temperature, using synthetic carbon-13 diamond, were developed and calibrated for routine use. Methods have also been developed with which to employ vibrational spectroscopic methods to determine stable isotope fractionation (principally D/H) between melts and coexisting fluids at high temperature and pressure.
The evolution of fluids speciation in different environments, especially under high-temperature/high-pressure conditions inside planets has been studied with molecular dynamics and thermodynamics modeling and by using spectroscopic techniques in experiments with the hydrothermal diamond anvil cell by CoI Mysen and collaborators Chi Zhang and Yingwei Fei. The preliminary results lead to the suggestion that the capacity of C-O-H fluid to hold carbon is decreasing when it is ascending from interior and carbon may precipitate through a disproportionation reaction among different carbon species.
Solubility and solution behavior of COH fluids in coexisting silicate melts and fluids, and C and H isotope fractionation between melt and fluid in silicate-COH systems have been determined in the 1-2.5 GPa pressure range at upper mantle temperatures and with hydrogen fugacity ranging from those defined by the hematite-magnetite + H2O [fH2(HM)] to iron-wüstite +H2O [fH2(IW)] buffers. Melt compositions were chosen to cover the degree of polymerization of their melt equivalent to that of natural melts ranging from basalt to andesite.
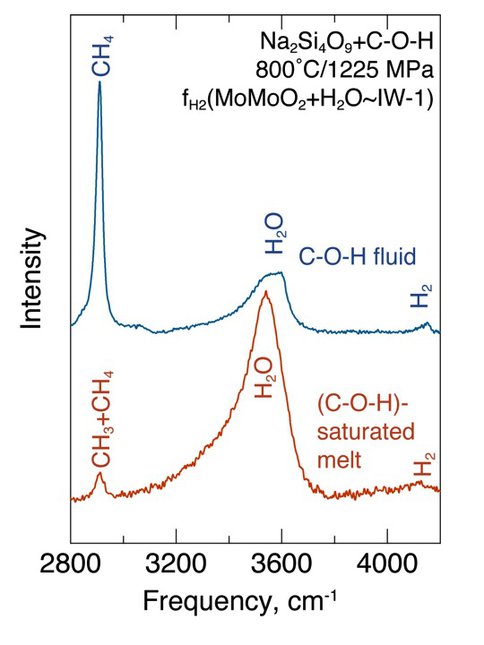
Solubility and solution mechanisms of COH volatiles in silicate melts and coexisting, silicate-saturated aqueous fluids were examined by CoIs Mysen, Cody, and Fogel together with collaborators Shigeru Yamashita (Univ. Okayama, Japan), and undergraduate summer intern Kathryn Kumamoto (Williams College). Methane solubility in melts, calculated as CH4, increases from 0.2 wt% to 0.5 wt% in the composition range between haploandesite and haplobasalt. The solubility increases by ~150% between the IW and MH oxygen buffers at constant temperature and pressure. At redox conditions of the NNO oxygen buffer and more oxidizing, carbon exists in melts as carbonate complexes and in the fluid as CO2. Reduced (C+H)-bearing species were detected in silicate melts at the redox conditions of the MW buffer and more reducing. From diamond cell (HDAC) experiments conducted in-situ from ambient temperature and pressure to 800 °C and 1435 MPa under redox conditions near those of the IW buffer, the dominant fluid species in the fluid are CH4, H2, and H2O. In coexisting melt, CH3 – groups linked to the silicate melt structure via Si-CH3 bonding coexist with molecular CH4 (Fig. 3.1) There is no evidence of changes in hydrocarbon species or polymerization with temperature and pressure. Carbon isotope fractionation between methane-saturated melts and (CH4+H2+H2O)-fluid changes by 14 ‰ at 1400 °C and 1.5 GPa in the composition range between haploandesite and haplobasalt. This change is positively correlated with the changes in the ratio of structurally-bound CH3 groups to molecular CH4 under conditions where fluid/melt mass ratio, fluid speciation, and species abundance are constant. The fH2-dependent solution mechanisms also affect silicate melt structure differently thus causing fH2-dependent thermodynamic and transport properties of magmatic liquids in the interior of the Earth and terrestrial planets. These properties include mineral melt minor and trace element partitioning, transport and thermodynamic properties of melt saturated with variably-oxidized COH volatile components.
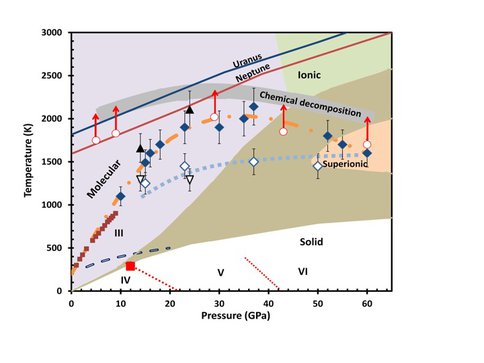
The role of reduced volatiles in the interior of large planets has been subjected to experimental study by CoI Goncharov together with postdoctoral collaborators Ojwang and McWilliams because the behavior of ammonia at high temperature and pressure is important for understanding the structure, gravitational moments, atmospheric composition, and magnetic field of giant planets where it is a major constituent. With the aid of a combination of in situ Raman spectroscopy and synchrotron x-ray diffraction in the laser-heated diamond anvil cell at pressures of 10 to 60 GPa and temperatures up to 2200 K – conditions directly relevant to the deep interiors of giant planets – we find a strong increase in the chemical reactivity of fluid ammonia, leading to the formation of molecular nitrogen and hydrogen and depletion of ammonia as shown in the Figure 3.2. These results suggest a potential deep reservoir of molecular nitrogen in giant planets, providing an alternative explanation for the presence of nitrogen in the atmospheres of giant planets such as Neptune.
We have also found it feasible to determine D/H fractionation between different phases and between individual function groups within a phase (fluid or melt), using NMR and vibrational spectroscopy, of samples both on quenched materials and while at high temperature and pressure in the hydrothermal diamond anvil cell. For example, during the last funding period CoI’s Cody and Mysen with NAI postdoctoral fellow Wang began experiments exploring the use of deuterium solid-state NMR spectroscopy to better understand the nature of water in silicate melts. It is well known that water has a profound effect on silicate melt properties and, in general, has a very high affinity for partioning into silicate melts where it resides in a number of different sites. Experiments have been conducted in quenched melts equilibrated with H2O+D2O fluids at 1.5 GPa and 1400 °C with the aid of deuterium and proton NMR. Remarkably, it was discovered that the deuterium very strongly partitions into certain sites while hydrogen favors other sites. The fractionation factor between certain sites can be as high as 25.
Experiments have been conducted to determine D/H fractionation between coexisting H2O-saturated silicate melt and silicate-saturated aqueous fluid using the hydrothermal diamond anvil cell using Raman spectroscopy to determine D/H ratios by CoI Mysen and collaborator Foustoukos. Experiments were conducted in the H2-D2-D2O-H2O fluid system at temperatures ranging from 300 – 800°C and pressures ~ 0.3 – 1.3 GPa in a hydrothermal diamond anvil cell, utilizing Raman spectroscopy as a quantitative tool to explore the relative distribution of hydrogen and deuterium isotopologues of the H2 and H2O in supercritical fluids. There are significant differences from the theoretical estimates of the equilibrium thermodynamic properties of the H-D exchange reactions. The exothermic behavior of the exchange reaction would enhance the stability of H2 and D2 relative to HD. Accordingly, the significant energy difference of the internal H2(aq)-D2(aq)-HD(aq) equilibrium translates to strong differences of the fractionation effects between the H2O-H2 and D2O-D2 isotope exchange relationships. The D/H isotope fractionation factors between H2O-H2(aq) and D2O-D2(aq) differ by 363 ‰ in the 600 – 800°C temperature range, and are indicative of the greater effect of D2O contribution to the dD isotopic composition of supercritical fluids.
3.5 Terrestrial Evolution
3.5.1 Geotectonics and carbon from deep mantle
The goal of this task, undertaken by Co-investigator Shirey, is to extend the understanding surface geological processes seen on Earth’s continents through the 150 km depths of their continental lithospheric mantle keels into the convecting mantle below and back in time. Key in this endeavor is the use of natural macro-diamonds and eclogites (high pressure meta-basalts) carried to the surface in kimberlitic magma. We study both the composition of diamonds and the age and composition of the diamond-hosted inclusions to follow the pathways of deeply sourced carbon and the origin of diamond-forming fluids. Such recycling is a fundamental geodynamic process that has occurred on Earth in some form since it accreted.
Stable, compositionally differentiated continental surfaces are topographically 5 km higher than oceanic lithospheric surfaces and start to be produced on Earth around 4 billion years ago. Prior to the existence of continents, the Earth’s lithosphere was oceanic and it is thought Earth’s heat was released primarily at upwelling oceanic ridges where hydrothermal circulation was active. If life was created prior to continents, it was there and was intimately connected to this early recycling process. The creation of continents presents the classic example of how planetary differentiation produces new types of habitat for subaerial life. But we do not know if subduction, in the form it exists today, or some other geodynamic process created the first continental crust on Earth. Whatever process existed, it was the dominant way for surface material and volatiles to become recycled into the Earth’s interior where they could have led to life harbored within the crust. How diamond-forming fluids access the continental lithosphere has potential importance to understanding the source of the crustal fluids that support subsurface microbial ecosystems. In addition, the tectonic processes that deliver these fluids play a role in making continents emergent surfaces that then create new substrates for life.
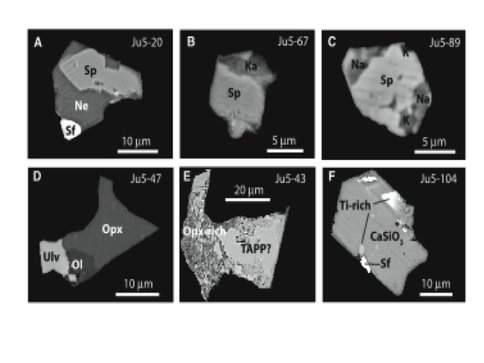
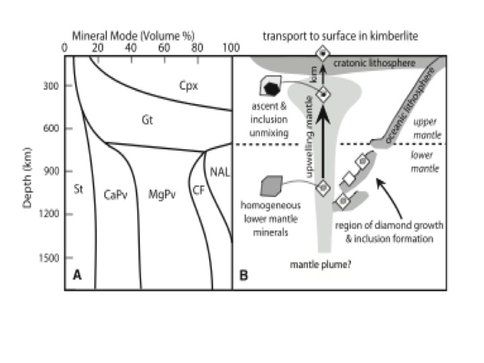
To look at the ability of the current subduction process to recycle material into the deep mantle, we analyzed a suite of superdeep low nitrogen (Type IIa) diamonds from the Juina-5 kimberlite, Brazil located in the Proterozoic Rio Negro-Juruena mobile belt southwest of the Amazon craton. These diamonds host inclusions with compositions comprising the phase assemblages expected to crystallize from basalt under lower mantle conditions (Fig. 3.3). Individual inclusions unmixed at lower pressures but can be ascribed to high pressure phases such as “calcium ferrite” (CF), the “new aluminum phase” (NAL), Al-, Ti- and Fe-rich Mg-perovskite, and Ti- rich Ca-perovskite. These phases exist only below 700 km depths in the lower mantle (Fig. 3.3). The inclusion mineralogies require exhumation from the lower to upper mantle which is hypothesized to occur in two stages: 1) upwelling in the 105 million year old Trinidade plume and 2) transport through the lithosphere to the surface in a kimberlitic magma (Fig. 3.4). Carbon isotopic compositions of the host diamonds are quite variable but 4 diamonds have isotopically light carbon isotopic signatures (δ13C = -15.4 to -24.1) not seen before in lower mantle diamonds. The most plausible hypothesis is that much of the carbon composing the diamonds derives from isotopically light, organic carbon that is present within altered basaltic oceanic crust on the sea floor. A primary consequence of plate tectonics is that basaltic oceanic crust subducts with lithospheric slabs into the mantle. Seismological studies have extended this process to the lower mantle, and geochemical observations from the basalts erupting on ocean islands indicate return of oceanic crust to the upper mantle in plumes. Until now, there has been no direct petrologic evidence of the return of subducted oceanic crustal components from the lower mantle. Because of these basaltic inclusions have diamond hosts with carbon isotopic signatures consistent with surface-derived carbon, this study leads to the important conclusion that the carbon cycling extends quite deep and into the lower mantle.
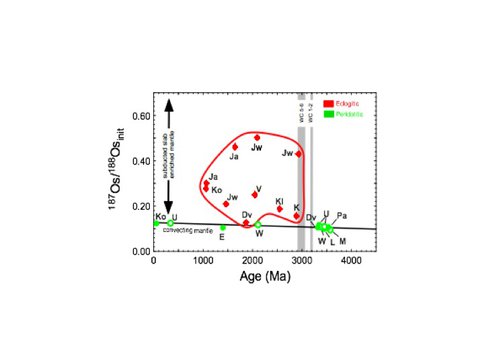
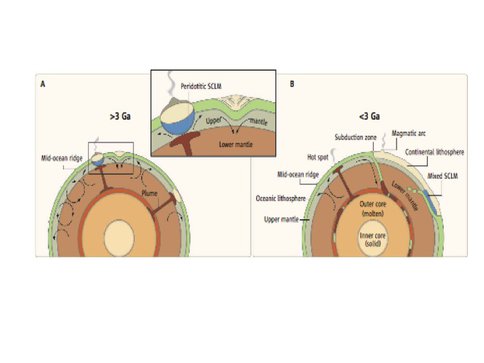
To look at the role of eclogite in tracking the onset of the subduction process on Earth, we complied geochronological studies of sulfide and silicate inclusions in diamonds from more than 20 kimberlites on 4 cratons that characterize the subcontinental lithospheric mantle (SCLM) through time (Fig. 3.5). Diamonds >3.2 Ga contain exclusively peridotitic (harzburgitic) silicate and sulfide inclusions whereas diamonds <3.0 Ga contain inclusions that are predominantly eclogitic. Similarly, >3.0 Ga kimberlite-borne eclogite xenoliths are largely absent in the SCLM rock record, whereas they are common thereafter. The lack of early eclogite implies an absence of steep slab subduction. Likewise, Archean crust records major differences across the 3.0–3.2 Ga interval. Prior to 3.2 Ga, crust grew by vertical accretion over upwelling mantle in long-lived plateaux floored by extremely depleted residual harzburgitic SCLM or via slab melting and crustal imbrication over shallow subduction zones (e.g West Greenland), whereas lateral accretion, allochthonous greenstone belt growth and calcalkaline magmatic products of mantle wedge melting emerge only after 3.2 Ga. This temporal and geochemical change can be explained as the result of a step-wise change in the tectonic style from rapid mantle convection, small plates, shallow subduction, and localized recycling >3.2 Ga, followed by large plates, steep subduction, and full upper mantle recycling <3.0 Ga (Fig. 3.6). These geodynamic changes had profound effects on mantle depletion, crustal growth, and geochemical cycles. This age has been proposed as a boundary between different geodynamic regimes on Earth and the start of the plate tectonic supercontinent (Wilson) cycle.
Our ongoing work will continue to use the diamond age evidence to explore the continent forming process from the bottom up, to synthesize available trace element and isotopic evidence from the literature to establish the onset of the subduction part of the plate tectonic cycle, and to examine the process of deep mantle recycling as evidenced in the diamond record. Study of diamonds will continue to be undertaken with secondary ion mass spectrometry (SIMS) and light spectroscopy (Raman, IR, CL) while study of the inclusions will continue to be undertaken with radioisotopic methods (Re-Os radiometric system on single sulfide inclusions).
3.5.2 Tectonics and Mineral Evolution
Mineral evolution, which frames mineralogy in an historical context, is based on the premise that the geo- and biospheres have coevolved through a sequence of deterministic and stochastic events. Three eras of mineral evolution – (1) planetary accretion, (2) crust and mantle reworking, and (3) biologically mediated mineralogy – each saw dramatic changes in the diversity and distribution of Earth’s near surface minerals. An important implication of this model is that different terrestrial planets and moons achieve different stages of mineral evolution. From a planetary perspective, the concept of mineral evolution allows each terrestrial body in the solar system to be placed in a broader mineralogical context. Mineral evolution provides an intellectual framework for identifying mineralogical targets in the search for extraterrestrial life.
Our efforts in mineral evolution research are now devoted to the detailed study of individual elements (Be, B, Mo, Hg) and mineral groups (clays, carbonates). Results for mercury (Hg) mineral evolution reveal striking trends that reflect significant geotectonic events. Our analyses of the temporal and geographic distribution of earliest recorded appearances of the 88 IMA approved mercury minerals plus 2 potentially valid species exemplify principals of mineral evolution. Metacinnabar (HgS) and native Hg are the only two species reported from meteorites, specifically, the primitive H3 Tieschitz chondrite with an age of 4550 Ma. Since the first terrestrial appearance of cinnabar more than 3 billion years ago, mercury minerals have been present continuously at or near Earth’s surface (Figure 3.7).
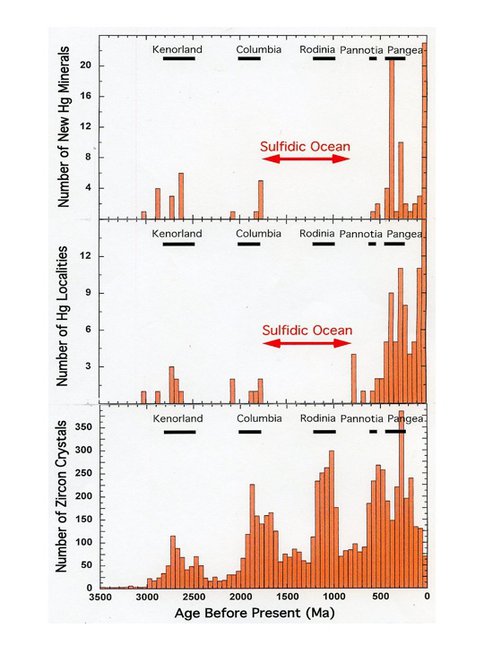
Mercury mineral evolution is characterized by episodic deposition and diversification, perhaps associated with the supercontinent cycle. We observe significant increases in the number of reported Hg mineral localities and new Hg species at ~2.8-2.6, ~1.9-1.8, and ~0.43-0.25 Ga – intervals that correlate with episodes of presumed supercontinent assembly and associated orogenies of Kenorland (Superia), Columbia (Nuna), and Pangea, respectively (Figure 3.8). In constrast, few Hg deposits or new species of mercury minerals are reported from the intervals of supercontinent stability and breakup at ~2.5-1.9, ~1.8-1.2, and 1.1-0.8 Ga. The interval of Pangean supercontinent stability and breakup (~250-65 Ma) is also marked by a significant decline in reported mercury mineralization; however, rocks of the last 65 million years, during which Pangea has continued to diverge, is characterized by numerous ephemeral near-surface Hg deposits.
The period ~1.2-1.0 Ga, during the assembly of the Rodinian supercontinent, is an exception because of the absence of new Hg minerals or deposits from this period (Figure 3.9). Episodes of Hg mineralization reflect metamorphism of Hg-enriched marine black shales at zones of continental convergence. We suggest that Hg was effectively sequestered as insoluble nanoparticles of cinnabar (HgS) or teimannite (HgSe) during the period of the sulfidic “intermediate ocean” (~1.85-0.85 Ga); consequently, few Hg deposits formed during the aggregation of Rodinia, whereas several deposits date from 800-600 Ma, a period that overlaps with the rifting and breakup of Rodinia.
Nearly all Hg mineral species (87 of 90 known), as well as all major economic Hg deposits, occur in formations ≤400 million years old. This relatively recent diversification arises, in part, from the ephemeral nature of many Hg minerals. In addition, mercury mineralization is strongly enhanced by interactions with organic matter, so the relatively recent pulse of new Hg minerals may reflect the rise of a terrestrial biosphere at ~400 Ma.
Figure 3.1 – Raman spectra of coexisting melt and fluid recorded in-situ in a hydrothermal diamond anvil cell at conditions indicated on the diagram.
Figure 3.2 – The phases proposed by Cavazonni et al. 3 (solid, molecular, ionic and superionic) are indicated by different colors; the brown region shows the error bar on the predicted phase boundaries. The Uranus and Neptune isentropes are from Ref. 4. Filled triangles and diamonds represent synchrotron XRD and Raman measurements of the melting line in this study, respectively. The open triangles and diamonds indicate the observed solid-solid phase transitions in our synchrotron XRD and Raman studies, respectively (fitted with a dotted blue line). The open circles indicate the temperature prior to the appearance of flash. The chemical decomposition band associated with flash, appearance of N2 and H2 in bulk quantities and polymorphic modifications of NH3 on quenching, is indicated with the thick gray line. The error bars in our measured T reflect the uncertainties (~200 K). The uncertainty in P is ~ 2 GPa. Our results for the melting line (the dashed-dotted orange line) is a fit of the Kechin melt equation: Tm(P) = T0(1 +P/a)be-cP, where a = 3.051 GPa, b=1.466, c = 0.039 GPa-1, T0 = 200 K and P is in units of GPa. The solid squares, the dashed line and the red square indicate the previously reported melting points, (IV,V)-III transition line and the IV-V transition point, respectively (data taken from Ref. 5); the indicated IV, V and VI phase boundaries (dotted red lines) are approximations.
Fig. 3.3. Backscattered electron micrographs showing composite inclusions in diamonds from Juina-5. (A) An inclusion in diamond Ju5-20 composed of a mixture of spinel (Mg,Fe)Al2O4 (Sp) and nepheline NaAlSiO4 (Ne) (fig. S1 and table S5), together with a small sulfide (Sf) in one corner that we interpret as an originally distinct phase from the composite silicate; sulfide can participate in diamond crystallization reactions as a melt phase that is immiscible in silicate (33). (B) An inclusion in diamond Ju5-67 that is composed of phases with the compositions of spinel and a nepheline-kalsilite (Ka) phase, (Na,K)AlSiO4 (table S5). (C) An inclusion in diamond Ju5-89 containing spinel and a mixture of micrometer-sized Na-rich (Na) and K-rich (K) silicate regions, with a bulk composition similar to Ju5-67 (fig. S2 and table S5). (D) An inclusion in diamond Ju5-47 that consists of orthopyroxene (Opx), ulvospinel (Ulv), and olivine (Ol) (fig. S3 and table S5). (E) An inclusion in diamond Ju5-43 that consists of a complex mixture of orthopyroxene and a Ti-, Al-, and Fe-rich phase similar to tetragonal almandine pyrope phase (TAPP) (table S5). (F) An inclusion in diamond Ju5-104 composed of CaSiO3 plus micrometer-sized Ti-rich phases (e.g., CaTiO3) and a small sulfide. Original figure and caption from Walter et al., Science 334:54-57
Fig. 3.4. (A) Estimated modal mineralogy in subducted basaltic oceanic crust as a function of depth in the mantle (17, 18). MgPv, Mg-perovskite; CaPv, Ca-perovskite; CF, CF phase; NAL, NAL phase; St, stishovite; Gt, garnet; Cpx, clinopyroxene. The inclusion mineralogy in diamonds from Juina-5, including MgPv, CaPv, CF phase, NAL phase, and stishovite, is stable at depths of ~700 to 1400 km in the lower mantle. (B) A schematic model for diamond formation and ascent beneath the Brazilian lithosphere. We suggest that the diamonds and inclusions initially formed from subducted oceanic crustal components in the upper part of the lower mantle and were transported in an upwelling plume to the upper mantle, where they unmixed into composite inclusions according to lower-pressure phase relations. Original figure and caption from Walter et al., Science 334:54-57
Fig. 3.5. Sulfide inclusion initial Os isotopic composition versus Re-Os age. WC 1-2 denotes Wilson cycle rifting (stages 1 and 2) for the Pilbara craton (6); WC 5-6 denotes Wilson cycle continental closure (stages 5 and 6) for the Kaapvaal craton.The presence eclogitic inclusions, formed from subducted basaltic material, only after the onset of the Wilson Cycle pinpoints the start of modern plate tectonics. From Shirey and Richardson, Science (2011) 333, 434-436.
Fig. 3.6. Schematic cross sections of (A) early Earth (>3 Ga) and (B) young Earth (<3 Ga), showing different modes of crust formation. On early Earth, crust formed in two settings (see enlargement): (i) over upwelling hot mantle, where it formed thick crust with a depleted keel of peridotitic subcontinental litho- spheric mantle (SCLM); (ii) over areas of downgoing mantle, where oceanic lithosphere was imbricated and extensively melted to form high-grade gneiss terrains. On young Earth with larger plates, whole- mantle convection generated steep subduction zones and the return of oceanic lithosphere to the mantle. Crust grew via subduction-generated arc magmatism and at hot spots. Subcretion of oceanic lithosphere to the base of continents resulted in SCLM with mixed peridotitic and eclogitic compositions. Figure and caption from Van Kranendonk, Science (2011) 333, 413, a perspectives article written about the results shown in Fig. 3.5.
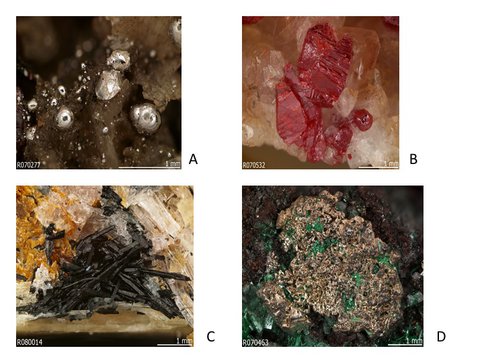
Figure 3.7. The diversity of mercury minerals reflects the variety of crystal chemical environments of Hg. (a) native mercury (Hg), RRUFF 070277, in massive and drusy quartz from the Socrates mine, Sonoma County, California, USA; (b) cinnabar (HgS), RRUFF 070532, on calcite from Charcas, San Luis Potosi, Mexico; (c) imiterite (Ag2HgS2), RRUFF 080014, with calcite and dolomite from Imiter, Morocco; (d) silver var. amalgam (Ag,Hg), RRUFF 070463, with malachite and calcite, from the Tsumeb mine, Namibia.
Figure 3.8. A histogram of the number of new mercury minerals (top) and Hg mineral localities (middle) versus time (50 million year bins) reveals pulses of mercury mineralization that correlate with three periods of supercontinent assembly. These episodes of Hg mineralization correlate with some, but not all, periods of increased zircon formation [(bottom)].
Publications
-
Anderson, B. J., Johnson, C. L., Korth, H., Purucker, M. E., Winslow, R. M., Slavin, J. A., … Zurbuchen, T. H. (2011). The Global Magnetic Field of Mercury from MESSENGER Orbital Observations. Science, 333(6051), 1859–1862. doi:10.1126/science.1211001
-
Aulbach, S., Stachel, T., Heaman, L. M., Creaser, R. A., & Shirey, S. B. (2010). Formation of cratonic subcontinental lithospheric mantle and complementary komatiite from hybrid plume sources. Contrib Mineral Petrol, 161(6), 947–960. doi:10.1007/s00410-010-0573-4
-
Baker, D. M. H., Head, J. W., Schon, S. C., Ernst, C. M., Prockter, L. M., Murchie, S. L., … Strom, R. G. (2011). The transition from complex crater to peak-ring basin on Mercury: New observations from MESSENGER flyby data and constraints on basin formation models. Planetary and Space Science, 59(15), 1932–1948. doi:10.1016/j.pss.2011.05.010
-
Blewett, D. T., Chabot, N. L., Denevi, B. W., Ernst, C. M., Head, J. W., Izenberg, N. R., … Hurwitz, D. M. (2011). Hollows on Mercury: MESSENGER Evidence for Geologically Recent Volatile-Related Activity. Science, 333(6051), 1856–1859. doi:10.1126/science.1211681
-
Chambers, J. E. (2010). STELLAR ELEMENTAL ABUNDANCE PATTERNS: IMPLICATIONS FOR PLANET FORMATION. The Astrophysical Journal, 724(1), 92–97. doi:10.1088/0004-637x/724/1/92
-
Ebel, D. S., & Alexander, C. M. O. D. (2011). Equilibrium condensation from chondritic porous IDP enriched vapor: Implications for Mercury and enstatite chondrite origins. Planetary and Space Science, 59(15), 1888–1894. doi:10.1016/j.pss.2011.07.017
-
Fassett, C. I., Kadish, S. J., Head, J. W., Solomon, S. C., & Strom, R. G. (2011). The global population of large craters on Mercury and comparison with the Moon. Geophysical Research Letters, 38(10), n/a–n/a. doi:10.1029/2011gl047294
-
Foustoukos, D. I., & Mysen, B. O. (2012). D/H fractionation in the H2–H2O system at supercritical water conditions: Compositional and hydrogen bonding effects. Geochimica et Cosmochimica Acta, 86, 88–102. doi:10.1016/j.gca.2012.03.003
-
Golden, J., McMillan, M., Downs, R. T., Hystad, G., Goldstein, I., Stein, H. J., … Hazen, R. M. (2013). Rhenium variations in molybdenite (MoS2): Evidence for progressive subsurface oxidation. Earth and Planetary Science Letters, 366, 1–5. doi:10.1016/j.epsl.2013.01.034
-
Goncharov, A. F., Subramanian, N., Ravindran, T. R., Somayazulu, M., Prakapenka, V. B., & Hemley, R. J. (2011). Polymorphism of dense, hot oxygen. J. Chem. Phys., 135(8), 084512. doi:10.1063/1.3626860
-
Gurney, J. J., Helmstaedt, H. H., Richardson, S. H., & Shirey, S. B. (2010). Diamonds through Time. Economic Geology, 105(3), 689–712. doi:10.2113/gsecongeo.105.3.689
-
Gómez-Pérez, N., & Solomon, S. C. (2010). Mercury’s weak magnetic field: A result of magnetospheric feedback?. Geophysical Research Letters, 37(20), n/a–n/a. doi:10.1029/2010gl044533
-
Hazen, R. M., Golden, J., Downs, R. T., Hystad, G., Grew, E. S., Azzolini, D., & Sverjensky, D. A. (2012). Mercury (Hg) mineral evolution: A mineralogical record of supercontinent assembly, changing ocean geochemistry, and the emerging terrestrial biosphere. American Mineralogist, 97(7), 1013–1042. doi:10.2138/am.2012.3922
-
Head, J. W., Chapman, C. R., Strom, R. G., Fassett, C. I., Denevi, B. W., Blewett, D. T., … Nittler, L. R. (2011). Flood Volcanism in the Northern High Latitudes of Mercury Revealed by MESSENGER. Science, 333(6051), 1853–1856. doi:10.1126/science.1211997
-
Janney, P. E., Shirey, S. B., Carlson, R. W., Pearson, D. G., Bell, D. R., Le Roex, A. P., … Boyd, F. R. (2010). Age, Composition and Thermal Characteristics of South African Off-Craton Mantle Lithosphere: Evidence for a Multi-Stage History. Journal of Petrology, 51(9), 1849–1890. doi:10.1093/petrology/egq041
-
Kerber, L., Head, J. W., Blewett, D. T., Solomon, S. C., Wilson, L., Murchie, S. L., … Domingue, D. L. (2011). The global distribution of pyroclastic deposits on Mercury: The view from MESSENGER flybys 1–3. Planetary and Space Science, 59(15), 1895–1909. doi:10.1016/j.pss.2011.03.020
-
Lawrence, D. J., Harmon, J. K., Feldman, W. C., Goldsten, J. O., Paige, D. A., Peplowski, P. N., … Solomon, S. C. (2011). Predictions of MESSENGER Neutron Spectrometer measurements for Mercury’s north polar region. Planetary and Space Science, 59(13), 1665–1669. doi:10.1016/j.pss.2011.07.001
-
Litasov, K. D., Goncharov, A. F., & Hemley, R. J. (2011). Crossover from melting to dissociation of CO2 under pressure: Implications for the lower mantle. Earth and Planetary Science Letters, 309(3-4), 318–323. doi:10.1016/j.epsl.2011.07.006
-
McCubbin, F. M., Jolliff, B. L., Nekvasil, H., Carpenter, P. K., Zeigler, R. A., Steele, A., … Lindsley, D. H. (2011). Fluorine and chlorine abundances in lunar apatite: Implications for heterogeneous distributions of magmatic volatiles in the lunar interior. Geochimica et Cosmochimica Acta, 75(17), 5073–5093. doi:10.1016/j.gca.2011.06.017
-
Mysen, B. (2010). Structure of H2O-saturated peralkaline aluminosilicate melt and coexisting aluminosilicate-saturated aqueous fluid determined in-situ to 800°C and ∼800MPa. Geochimica et Cosmochimica Acta, 74(14), 4123–4139. doi:10.1016/j.gca.2010.04.024
-
Mysen, B. (2012). High-pressure and high-temperature titanium solution mechanisms in silicate-saturated aqueous fluids and hydrous silicate melts. American Mineralogist, 97(7), 1241–1251. doi:10.2138/am.2012.4084
-
Mysen, B. O. (2010). Speciation and mixing behavior of silica-saturated aqueous fluid at high temperature and pressure. American Mineralogist, 95(11-12), 1807–1816. doi:10.2138/am.2010.3539
-
Mysen, B. O. (2011). Amorphous Materials: An experimental study of phosphorous and aluminosilicate speciation in and partitioning between aqueous fluids and silicate melts determined in-situ at high temperature and pressure. American Mineralogist, 96(10), 1636–1649. doi:10.2138/am.2011.3728
-
Mysen, B. O., & Yamashita, S. (2010). Speciation of reduced C–O–H volatiles in coexisting fluids and silicate melts determined in-situ to ∼1.4GPa and 800°C. Geochimica et Cosmochimica Acta, 74(15), 4577–4588. doi:10.1016/j.gca.2010.05.004
-
Mysen, B. O., Kumamoto, K., Cody, G. D., & Fogel, M. L. (2011). Solubility and solution mechanisms of C–O–H volatiles in silicate melt with variable redox conditions and melt composition at upper mantle temperatures and pressures. Geochimica et Cosmochimica Acta, 75(20), 6183–6199. doi:10.1016/j.gca.2011.07.035
-
Nittler, L. R., Starr, R. D., Weider, S. Z., McCoy, T. J., Boynton, W. V., Ebel, D. S., … Sprague, A. L. (2011). The Major-Element Composition of Mercury’s Surface from MESSENGER X-ray Spectrometry. Science, 333(6051), 1847–1850. doi:10.1126/science.1211567
-
Oberst, J., Elgner, S., Turner, F. S., Perry, M. E., Gaskell, R. W., Zuber, M. T., … Solomon, S. C. (2011). Radius and limb topography of Mercury obtained from images acquired during the MESSENGER flybys. Planetary and Space Science, 59(15), 1918–1924. doi:10.1016/j.pss.2011.07.003
-
Peplowski, P. N., Evans, L. G., Hauck, S. A., McCoy, T. J., Boynton, W. V., Gillis-Davis, J. J., … Stockstill-Cahill, K. R. (2011). Radioactive Elements on Mercury’s Surface from MESSENGER: Implications for the Planet’s Formation and Evolution. Science, 333(6051), 1850–1852. doi:10.1126/science.1211576
-
Perry, M. E., Kahan, D. S., Barnouin, O. S., Ernst, C. M., Solomon, S. C., Zuber, M. T., … Asmar, S. W. (2011). Measurement of the radius of Mercury by radio occultation during the MESSENGER flybys. Planetary and Space Science, 59(15), 1925–1931. doi:10.1016/j.pss.2011.07.022
-
Preusker, F., Oberst, J., Head, J. W., Watters, T. R., Robinson, M. S., Zuber, M. T., & Solomon, S. C. (2011). Stereo topographic models of Mercury after three MESSENGER flybys. Planetary and Space Science, 59(15), 1910–1917. doi:10.1016/j.pss.2011.07.005
-
Rhodes, E. A., Evans, L. G., Nittler, L. R., Starr, R. D., Sprague, A. L., Lawrence, D. J., … Solomon, S. C. (2011). Analysis of MESSENGER Gamma-Ray Spectrometer data from the Mercury flybys. Planetary and Space Science, 59(15), 1829–1841. doi:10.1016/j.pss.2011.07.018
-
Schon, S. C., Head, J. W., Baker, D. M. H., Ernst, C. M., Prockter, L. M., Murchie, S. L., & Solomon, S. C. (2011). Eminescu impact structure: Insight into the transition from complex crater to peak-ring basin on Mercury. Planetary and Space Science, 59(15), 1949–1959. doi:10.1016/j.pss.2011.02.003
-
Shirey, S. B., & Richardson, S. H. (2011). Start of the Wilson Cycle at 3 Ga Shown by Diamonds from Subcontinental Mantle. Science, 333(6041), 434–436. doi:10.1126/science.1206275
-
Smit, K. V., Shirey, S. B., Richardson, S. H., Le Roex, A. P., & Gurney, J. J. (2010). Re–Os isotopic composition of peridotitic sulphide inclusions in diamonds from Ellendale, Australia: Age constraints on Kimberley cratonic lithosphere. Geochimica et Cosmochimica Acta, 74(11), 3292–3306. doi:10.1016/j.gca.2010.03.001
-
Solomon, S. C. (2011). A new look at the planet Mercury. Physics Today, 64(1), 50. doi:10.1063/1.3541945
-
Strom, R. G., Banks, M. E., Chapman, C. R., Fassett, C. I., Forde, J. A., Head, J. W., … Solomon, S. C. (2011). Mercury crater statistics from MESSENGER flybys: Implications for stratigraphy and resurfacing history. Planetary and Space Science, 59(15), 1960–1967. doi:10.1016/j.pss.2011.03.018
-
Subramanian, N., Goncharov, A. F., Struzhkin, V. V., Somayazulu, M., & Hemley, R. J. (2011). Bonding changes in hot fluid hydrogen at megabar pressures. Proceedings of the National Academy of Sciences, 108(15), 6014–6019. doi:10.1073/pnas.1102760108
-
Walter, M. J., Kohn, S. C., Araujo, D., Bulanova, G. P., Smith, C. B., Gaillou, E., … Shirey, S. B. (2011). Deep Mantle Cycling of Oceanic Crust: Evidence from Diamonds and Their Mineral Inclusions. Science, 334(6052), 54–57. doi:10.1126/science.1209300
-
Zhang, C., & Duan, Z. (2010). GFluid: An Excel spreadsheet for investigating C–O–H fluid composition under high temperatures and pressures. Computers & Geosciences, 36(4), 569–572. doi:10.1016/j.cageo.2009.05.008
-
Zhang, C., Duan, Z., & Li, M. (2010). Interstitial voids in silica melts and implication for argon solubility under high pressures. Geochimica et Cosmochimica Acta, 74(14), 4140–4149. doi:10.1016/j.gca.2010.04.017
- Anderson, B.J., Johnson, C.L., Korth, H., Purucker, M.E., Moldovan, R., Solomon, S.C. & McNutt, R.L., Jr. (2011). The global-scale magnetic field of Mercury. EPSC-DPS 2011. Nantes, France.
- Anderson, B.J., Korth, H., Slavin, J.A., Johnson, C.L., Purucker, M.E., Solomon, S.C., Raines, J.M., Zurbuchen, T.H., Gloeckler, G. & McNutt, R.L., Jr. (2010). Magnetospheric magnetic field observations and modeling at Mercury. Exploring Magnetosphere–Exopshere Coupling at Mercury: A Joint MESSENGER–BepiColombo Workshop. Boulder, Colo.
- Aulbach, S., Stachel, T., Heaman, L.M., Creaser, R.A. & Shirey, S.B. (2010). Formation of cratonic subcontinental lithospheric mantle from hybrid plume sources. Geochimica et Cosmichimica Acta, 74: A37.
- Aulbach, S., Stachel, T., Heaman, L.M., Creaser, R.A., Thomassot, E. & Shirey, S.B. (2011). C- and S-Transfer in Subduction Zones: Insights from Diamonds. Mineralogical Magazine, 75: 462.
- Baker, D.M.H., Head, J.W., Prockter, L.M. & Solomon, S.C. (2011). Mercury’s peak-ring basin populatioon and the formation of peak rings: Observations from MESSENGER flyby and orbital data. Abstracts with Programs, Geological Society of America, 43(5): paper 142-12, p. 359.
- Braden, S.E., Robinson, M.S., Denevi, B.W. & Solomon, S.C. (2011). Reflectance of Mercury and the Moon. EPSC-DPS 2011. Nantes, France.
- Byrne, P.K., Klimczak, C., Denevi, B.W., Watters, T.R., Solomon, S.C., Enns, A., Head, J.W., Hurwitz, D.M. & Baker, D.M.H. (2011). Surface lava flow features on Mercury. Abstracts with Programs, Geological Society of America, 43(5): paper 142-7, p. 358.
- Chapman, C.R., Merline, W.J., Ostrach, L.R., Xiao, Z., Solomon, S.C. & Head, J.W., III. (2011). Small craters (secondaries) on Mercury’s northern plains. EPSC-DPS 2011. Nantes, France.
- Chapman, C.R., Merline, W.J., Ostrach, L.R., Xiao, Z., Solomon, S.C., Head, J.W. & Whitten, J.L. (2011). Statistics of morphologies of small primary and secondary craters on Mercury’s northern plains. Abstracts with Programs, Geological Society of America, 43(5): paper 142-13, p. 359.
- D’Amore, M., Helbert, J., Maturilli, A., Domingue, D.L., Izenberg, N.R. & Solomon, S.C. (2011). Compositio of surface units on Mercury from surface reflectance measurements during the first and second MESSENGER flybys. Lunar and Planetary Science, 52: abstract 1381.
- D’Amore, M., Helbert, J., Maturilli, A., Sprague, A.L., Izenberg, N.R., Holsclaw, G.M., McClintock, W.E., Vilas, F. & Solomon, S.C. (2011). Surface compositional heterogeneity on Mercury inferred from MESSENGER spectral measurements. EPSC-DPS 2011. Nantes, France.
- Denevi, B.W., Robinson, M.S., Blewett, D.T., Murchie, S.L., Chabot, N.L., Ernst, C.M., Solomon, S.C. & Head, J.W. (2011). Origin and significance of plains on Mercury. EPSC-DPS 2011. Nantes, France.
- Ebel, D.S., Alexander, C.M.O.D., Hauck, S.A., II, Lawrence, D.J., Nittler, L.R., Peplowski, P.N., Solomon, S.C., Sprague, A.L., Starr, R.D. & Stewart, S.T. (2011). MESSENGER: Implications for Mercury formation hypotheses. Abstracts with Programs, Geological Society of America, 43(5): paper 142-8, p. 358.
- Ernst, C.M., Murchie, S.L., Barnouin, O.S., Chabot, N.L., Head, J.W., Prockter, L.M., Solomon, S.C. & Watters, T.R. (2011). Thickness of volcanic fill in impact basins on Mercury. Abstracts with Programs, Geological Society of America, 43(5): paper 142-8, p. 358.
- Evans, L.G., Peplowski, P.N., Hauck, S.A., II, McClintock, W.E., Boynton, W.V., Goldsten, J.O., Hamara, D.K., Rhodes, E.A., Sprague, A.L. & Solomon, S.C. (2011). Radioactive elements measured on Mercury by MESSENGER: Implications for the planet’s formation and evolution. Abstracts with Programs, Geological Society of America, 43(5): paper 142-4, p. 357.
- Fei, Y., Hillgren, V.J., Shahar, A. & Solomon, S.C. (2011). On the silicon content of Mercury’s core and implications for core mineralogy. Lunar and Planetary Science, 52: abstract 1949.
- Fei, Y., Zhang, C. & Tao, R. (2011). Efficient carbon leaching in silicate through fluid/melt migration and implications for diamond formation. Goldschmidt Conference. Prague, Czech REepublic.
- Foustoukos, D.I. & Mysen, B.O. (2011). D/H fractionation effects in the H2-H2O system: An in-situ experimental study at supercritical water conditions. American Geophysical Union, Fall Meeting. San Francisco.
- Goncharov, A.F. (2011). Raman Spectroscopy at High Pressures.
- Goncharov, A.F., Dalton, D.A., McWilliams, R.S., Armstrong, M.R. & Crowhurst, J.C. (2011). Development of new ultrafast laser techniques for diamond anvil cell research: current stage and future plans. SMEC 2011 Meeting, March 27-April 1, 2011. Miami – Belize – Mexico – Miami.
- Gómez-Pérez, N., Heyner, D., Wicht, J. & Solomon, S.C. (2010). Magnetospheric feedback effects on Mercury’s dynamo. 2010 Fall Meeting, American Geophysical Union. San Francisco, Calif.
- Hauck, S.A., II, Zuber, M.T., Smith, D.E., Johnson, C.L., Phillips, R.J., Lemoine, F.G., Margot, J-L., Neumann, G.A., Peale, S.J. & Solomon, S.C. (2011). The geophysics of Mercury: MESSENGER’s view from orbit. Abstracts with Programs, Geological Society of America (43): 5
- Hazen, R.M., Downs, R.T., Golden, J., Grew, E.S., McMillan, M., Ralph, J.P. & Sverjensky, D.A. (2011). Mineral evolution: What’s next? Goldschmidt Cinference, Prague Czech Republic.
- Helbert, J., D’Amore, M., Maturilli, A., Sprague, A.L., Izenberg, N.R., Holsclaw, G.M., Domingue, D.L., McClintock, W.E., Hiesinger, H. & Solomon, S.C. (2011). Bridging from MESSENGER to BepiColombo – surface composition from visual and near-infrared observations and simulation of thermal infrared data. EPSC-DPS 2011. Nantes, France.
- Izenberg, N.R., Holsclaw, G.M., Domingue, D.L., McClintock, W.E., Blewett, D.T., Kochte, M.C., Helbert, J., Sprague, A.L., Vilas, F. & Solomon, S.C. (2011). Surface reflectance of Mercury from MESSENGER orbital observations. EPSC-DPS 2011. Nantes, France.
- Klimczak, C., Watters, T.R., Byrne, P.K., Ernst, C.M., Solomon, S.C., Goudge, T.A., Head, J.W. & Xiao, Z. (2011). Strain analysis of extension in volcanically flooded impact craters on Mercury. Abstracts with Programs, Geological Society of America, 43(5): paper 142-10.
- Lawrence, D.J., Harmon, J.K., Feldman, W.C., Paige, D.A., Peplowski, P.N., Selby, C.M. & Solomon, S.C. (2011). Predictions of MESSENGER Neutron Spectrometer measurements for Mercury’s polar regions. Lunar and Planetary Science, 52: abstract 1955.
- Lemoine, F.G., Smith, D.E., Peale, S.J., Phillips, R.J., Solomon, S.C., Zuber, M.T., Margot, J-L., Neumann, G.A., Torrence, M.H. & Perry, M.E. (2011). Mercury’s gravity field from MESSENGER after 6 months in orbit. EPSC-DPS 2011. Nantes, France.
- Lin, J-F., Alp, E.E. & Goncharov, A.F. (2011). Raman and Nuclear Resonant Spectroscopy in Geosciences.
- McClintock, W.E., Burger, M.H., Killen, R.M., Merkel, A.W., Sarantos, M., Sprague, A.L., Solomon, S.C. & Vervack, R.J., Jr. (2011). Insights into the nature of Mercury’s exosphere: Early results from the MESSENGER orbital mission phase. EPSC-DPS 2011. Nantes, France.
- McCubbin, F.M., Sverjensky, D.A., Steele, A. & Mysen, B.O. (2011). In-situ characterization of oxalic acid breakdown at elevanted P and T: Implications for organic C-O-H fluid sources in petrologic experiments. Amer. Mineral, Submitted.
- McMillan, M.M., Downs, R.T., Stein, H.J., Zimmerman, A., Beitscher, B., Sverjensky, D.A., Papineau, D., Armstrong, J. & Hazen, R.M. (2010). Molybdenite mineral evolution: A study of trace elements through time. Geological Society of America.
- Moldovan, R., Johnson, C.L., Ritzer, J.A., Purucker, M.E., Solomon, S.C., Anderson, B.J., Denevi, B.W. & Korth, H. (2011). Detecting crustal magnetic fields on Mercury with MESSENGER. Lunar and Planetary Science, 52: Abstract 2481.
- Murchie, S.L., Robinson, M.S., Head, J.W., Strom, R.G., Prockter, L.M., Solomon, S.C., Watters, T.R., Blewett, D.T., Chabot, N.L. & Denevi, B.W. (2011). Results from MESSENGER’s first solar day of orbital imaging on Mercury. Abstracts with Programs, Geological Society of America, 43(5): paper 142-1, p. 357.
- Mysen, B.O. (2010). Speciation of P in and partitioning between aqueous fluids and silicate melts to upper mantle temperatures and pressures. JpGU 2011. Makuhari, Japan.
- Mysen, B.O. (2011). In-situ, high-pressure/-temperature experimental determination of structure-property relations in silicate melt-COHN systems. Goldschmidt Conference. Prague, Czech Republic.
- Mysen, B.O. (2011). P5+ and Ti4+ solution mechanisms of and partitioning between fluids and melts at crustal and upper mantle pressure and temperature. Goldschmidt Conference. Prague, Czech Republic.
- Mysen, B.O. (2011). Solubility and speciation of volatiles in melts coexisting with COHN fluids at high temperature and pressure with redox conditions: Influence on isotope fraction and melt properties. 9th International Conference on Silicate Melts. La Petite Pierre, France.
- Mysen, B.O., Fogel, M.L., Yamashita, S., Cody, G.D. & Kumamoto, K. (2010). Influence of speciation of C-O-H-N volatiles in silicate melts and coexisting fluids on C and N solubility in melts and on C and N isotope fractionation between melt and coexisting fluid to upper mantle pressure and temperature as a function of redox conditions. AGU Fall Meeting. San Francisco.
- Mysen, B.O., Kumamoto, K., Cody, G.D. & Fogel, M.L. (2010). COH Solubility, solution behavior and 13/12C fractionation melt-fluid systems at mantle redox, P, and T conditions. JpGU 2010. Makuhari, Japan.
- Nittler, L.R., Weider, S.Z., Starr, R.D., McClintock, W.E., Ebel, D.S., Lawrence, D.J., McNutt, R.L., Jr., Schlemmn, C.E., II, Solomon, S.C. & Sprague, A.L. (2011). Major elements on Mercury’s surface from MESSENGER X-ray spectrometry. Abstracts with Programs, Geological Society of America, 43(5).
- Ojwang, J., McWilliams, R. & Goncharov, A. (2011). High pressure—high temperature studies of ammonia. Bulletin of the American Physical Society, APS March Meeting 2011, Abstract ID: BAPS.2011.MAR.Q31.6.
- Ojwang, J.G.O., McWilliams, R.S. & Goncharov, A.F. (2011). Melting and Dissociation of Ammonia at High Pressures.
- Ostrach, L.R., Chapman, C.R., Fassett, C.I., Head, J.W., Merline, W.J., Robinson, M.S., Solomon, S.C., Strom, R.G. & Xiao, Z. (2011). Crater statistics for the northern polar region of Mercury derived from MESSENGER orbital data. Abstracts with Programs, Geological Society of America, 43(5): paper 142-14, p. 360.
- Paige, D.A., Siegler, M.A., Harmon, J.K., Smith, D.E., Zuber, M.T. & Solomon, S.C. (2011). Stability of ices in the north polar region of Mercury. EPSC-DPS 2011. Nantes, France.
- Peplowski, P.N., Evans, L.G., Blewett, D.T., Denevi, B.W., Lawrence, D.J., Nittler, L.R., Rhodes, E.A. & Solomon, S.C. (2011). Surface abundances of K, Th, and U on Mercury and implications for planet formation and evolution. Lunar and Planetary Science, 52: abstract 2290.
- Peplowski, P.N., Hamara, D.K., Evans, L.G., McClintock, W.E., Boynton, W.V., Lawrence, D.J., Nittler, L.R., Rhodes, E.A., Solomon, S.C. & Sprague, A.L. (2011). Potassium and thorium on the surface of Mercry: Early results from the MESSENGER Gamma-Ray Spectrometer. EPSC-DPS 2011. Nantes, France.
- Perry, M.E., Kahan, D.S., Barnouin, O.S., Ernst, C.M., Solomon, S.C., Zuber, M.T. & Smith, D.E. (2011). Mercury’s radis and shape from radio occultations. EPSC-DPS 2011. Nantes, France.
- Prockter, L.M., Baker, D.M.H., Head, J.W., Murchie, S.L., Ernst, C.M., Chabot, N.L., Denevi, B.W., Solomon, S.C., Watters, T.R. & Massironi, M. (2011). The geology of medium-sized impact basins on Mercury. Abstracts with Programs, Geological Society of America, 43(5): paper 142-11, p. 359.
- Purucker, M.E., Johnson, C.L., Moldovan, R., Zuber, M.T., Solomon, S.C., Anderson, B.J., Korth, H., Paige, D.A., Slavin, J.A., Alexeev, I.I. & Phillips, R.J. (2011). A search for the crustal magnetization siganture of variations in insolation at Mercury. EPSC-DPS 2011. Nantes, France.
- Sarantos, M., Killen, R.M., McClintock, W.E., Slavin, J.A., Solomon, S.C. & Vervack, R.J., Jr. (2011). Tomographic reconstruction of Mercury’s exosphere from Mercury flyby data. EPSC-DPS 2011. Nantes, France.
- Shirey, S.B., Richardson, S.H. & Van Kranendonk, M.J. (2011). 3 Ga Onset of the Supercontinent Cycle: SCLM and Crustal Evidence. Mineralogical Magazine, 75: 1863.
- Shirey, S.B., Richardson, S.H., Aulbach, S. & Pearson, D.G. (2010). Secular Changes in Lithospheric Diamonds from the Archean to the Proterozoic. EOS Transactions of the AGU, 91: U33A-0009.
- Solomon, S.C. (2011). A tale of two spacecraft: The exploration of Mercury. Abstracts with Programs, Geological Society of America, 43(5): paper 199-11, p. 487.
- Solomon, S.C. (2011). The exploration of Mercury by the MESSENGER spacecraft. GAC-MAC-SEG-SGA Joinit Annual Meeting. Ottawa, Ontario, Canada.
- Solomon, S.C., McNutt, R.L., Jr., Bedini, P.D., Anderson, B.J., Blewett, D.T., Evans, L.G., Gold, R.E., Krimigis, S.M., Murchie, S.L., Nittler, L.R., Phillips, R.J., Prockter, L.M., Slavin, J.A. & Zuber, M.T. (2011). MESSENGER at Mercury: Flyby accomplishments and orbital observing plans. Lunar and Planetary Science, 52: abstract 1781.
- Solomon, S.C., McNutt, R.L., Jr., Bedini, P.D., Anderson, B.J., Blewett, D.T., Evans, L.G., Gold, R.E., Krimigis, S.M., Murchie, S.L., Nittler, L.R., Phillips, R.J., Prockter, L.M., Slavin, J.A. & Zuber, M.T. (2011). Mercury after six months of MESSENGER orbital observations. EPSC-DPS 2011. Nantes, France.
- Solomon, S.C., McNutt, R.L., Jr., Bedini, P.D., Anderson, B.J., Prockter, L.M., Blewett, D.T., Evans, L.G., Gold, R.E., Krimigis, S.M., Murchie, S.L., Nittler, L.R., Phillips, R.J., Slavin, J.A. & Zuber, M.T. (2010). The exploration of Mercury by MESSENGER: Looking ahead to orbital observations. 2010 Fall Meeting, American Geophysical Union. San Francisco, Calif.
- Starr, R.D., Weider, S.Z., Nittler, L.R., Boynton, W.V., Evans, L.G., Goldsten, J.O., McClintock, W.E., McNutt, R.L., Jr., Schlemmn, C.E., II, Solomon, S.C. & Sprague, A.L. (2011). Mercury’s surface composition: Early results from the MESSENGER X-Ray Spectrometer. EPSC-DPS 2011. Nantes, France.
- Strom, R.G., Banks, M.E., Chapman, C.R., Fassett, C.I., Forde, J.A., Head, J.W., Merline, W.J., Prockter, L.M. & Solomon, S.C. (2011). Mercury crater statistics from MESSENGER flybys: Implications for stratigraphy and resurfacing history. Lunar and Planetary Science, 52: abstract 1079.
- Vilas, F., Domingue, D.L., Jensen, E.A., Sprague, A.L., Helbert, J., D’Amore, M. & Solomon, S.C. (2011). Search for absorption features in Mercury’s ultraviolet and visible reflectance properties. EPSC-DPS 2011. Nantes, France.
- Watters, T.R., Solomon, S.C., Head, J.W., Ernst, C.M., Denevi, B.W., Robinson, M.S., Klimczak, C. & Goudge, T.A. (2011). Extension in the northern plains of Mercury. Abstracts with Programs, Geological Society of America, 43(5): paper 142-9, p. 358.
- Yamashita, S. & Mysen, B.O. (2010). Pressure and temperature dependence of 13C diamond Raman shift determined in-situ to 1.27 GPa and 800 °C: application to pressure sensor for high temperature diamond anvil cell experiments. Japan Geophysical Union, Annual Meeting. Makuhari, Japan.
- Zhang, C., Fei, Y. & Mysen, B.O. (2011). In-situ characterization of speciation in C-O-H systems under high temperature and pressure. COMPRES Annual Meeting. Williamsburg, VA.
- Zuber, M.T., Phillips, R.J., Smith, D.E., Solomon, S.C., Neumann, G.A., Lemoine, F.G., Peale, S.J., Margot, J-L., Hauck, S.A., II, Head, J.W., Johnson, C.L., Purucker, M.E., Oberst, J., Barnouin, O.S., Perry, M.E. & Torrence, M.H. (2011). Initial orbital phase results of the MESSENGER geophysics investigation. Geophysical Research Abstracts, 13: abstract EGU2011-11251.
- Zuber, M.T., Smith, D.E., Phillips, R.J., Solomon, S.C., Neumann, G.A., Head, J.W., Torrence, M.H., Lemoine, F.G., Mazarico, E., Hauck, S.A., II, Johnson, C.L., Barnouin, O.S., Perry, M.E., Oberst, J., Yang, D. & Ernst, C.M. (2011). Orbital observations of Mercury with the Mercury Laser Altimeter. EPSC-DPS 2011. Nantes, France.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Marilyn Fogel
Co-Investigator
Douglas Rumble
Co-Investigator
Ed Grew
Collaborator
Penny Morrill
Collaborator
Mihaela Glamoclija
Postdoc
Konstantin Litasov
Postdoc
Francis McCubbin
Postdoc
Stewart McWilliams
Postdoc
Julius Ojwang
Postdoc
Thomas Reudas
Postdoc
Ying Wang
Postdoc
Chi Zhang
Postdoc
Dionysis Foustoukos
Research Staff
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 4.1
Earth's early biosphere.