2011 Annual Science Report
 Arizona State University
Reporting | SEP 2010 – AUG 2011
Arizona State University
Reporting | SEP 2010 – AUG 2011
Habitability of Water-Rich Environments, Task 5: Evaluate the Habitability of Small Icy Satellites and Minor Planets
Project Summary
We are investigating whether liquid can exist beneath the surface ice of small icy satellites and Kuiper belt objects (KBOs). We are also trying to predict whether this liquid can be brought to the surface in a “cryovolcanic” flow, or if there are other observational signatures of subsurface liquid. Numerical modeling has been performed to understand physical and chemical processes, fluid chemistry and mineralogy of low- and high-temperature aqueous processes on icy bodies in the outer solar system.
Project Progress
S. Desch with his group continued their work on thermal structure and evolution models of Kuiper belt objects (KBOs) and small icy satellites. The models have been updated to account for crystalline/amorphous ice transition and for Rayleigh-Taylor instabilities within the body. These models predict the likelihood of subsurface water and cryovolcanism on these bodies. A paper is being prepared for publication (Rubin & Desch 2012, in prep).
Chemical disequilibria inside the Saturnian icy moon Enceladus have been evaluated by M. Zolotov in the context of habitability and reactivity of organic species in putative aqueous environments. The comet-like abundances of major plume gases and apparent redox disequilibria in aquatic systems are consistent with a minimal influence of aqueous processes on endogenic chemical reactions and may indicate abiotic interior.
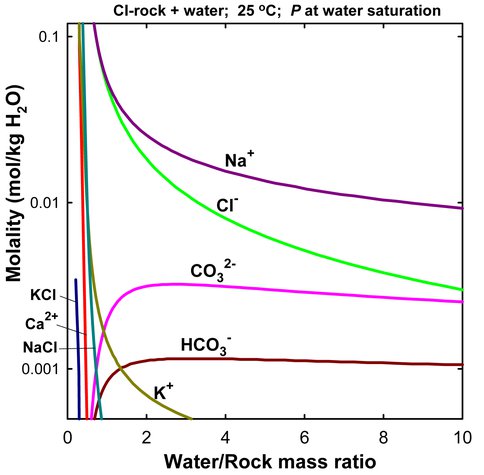
M. Zolotov used chemical equilibrium models to evaluate fluid composition and pH during aqueous alteration of CI carbonaceous chondritic materials, which may represent rocks in icy bodies. The results show that fluid chemistry is mainly affected by solubility of secondary minerals, concentration and speciation of Cl in initial water(ice)-rock mixtures, and degree of sulfide to sulfate conversion in oxidized conditions. If sulfates do not form, solutions are represented by NaCl-rich compositions with lesser amounts of HCO3-, CO32-, K+ and organic solutes. The models are consistent with the detection of NaCl-Na2CO3 bearing grains emitted from Enceladus.
Chemical processes responsible for formation and evolution of oceans on icy moons have been discussed in a review paper published in Space Science Reviews. Another review paper on chemistry of Enceladus (Zolotov et al.) is prepared for submission.
M. Zolotov participated in interpretation of Cassini Neutral and Ion Mass Spectrometer and Cosmic Dust Detector data obtained for plume emissions of Enceladus.
Graduate student Chris Glein is putting the finishing touches on a thermodynamic model to compute solubilities of gases and solids in liquid hydrocarbon lakes on the surface of Saturn’s moon Titan.
The modeled composition of aqueous fluid formed through alteration of the CI carbonaceous chondritic material (Zolotov, 2011). The solution is alkaline with pH is 9 to 11. This modeled composition is consisted with composition of salts emitted from the Saturnian icy moon Enceladus. The composition could be used as a proxy for oceanic chemistry of many icy moons. This solution could be easily prepared by dissolution of table salt and baking soda in water.
Publications
- Cook, J.C., Desch, S.J. & Rubin, M. (2011). The black sheep of Haumea’s Collisional Family. 42nd Lunar and Planetary Science Conference. Houston, TX.
- Waite, J.H., Magee, B., Brockwell, T., Zolotov, M., Yu, M., Teolis, B., Lewis, W.S. & Team, I. (2011). Enceladus’ plume composition. Joint European Planetary Science Congress and DPS meeting. Nantes, France.
- Zolotov, M. & Yu, M. (2010). Chemical disequilibria and sources of Gibbs Free Energy inside Enceladus. AGU. San Francisco, CA.
- Zolotov, M. & Yu, M. (2011). Fluid chemistry of aqueous alteration of C1-type chondritic materials: Thermodynamic assessment. 42nd Lunar and Planetary Science Conference. Houston, TX.
- Zolotov, M., Yu, M., Tobie, G., Postberg, F., Magee, B., Waite, J.H. & Esposito, L. (2011). Chemical and phase composition of Enceladus: Insights from Cassini observations. Joint European Planetary Science Congress and DPS meeting. Nantes, France.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Everett Shock
Co-Investigator
Allen McNamara
Collaborator
Karen Meech
Collaborator
Mikhail Mironenko
Collaborator
Jason Cook
Postdoc
Divya Allu Peddinti
Graduate Student
Christopher Glein
Graduate Student
Mark Rubin
Graduate Student
Sarah Sonnett
Graduate Student
-
RELATED OBJECTIVES:
Objective 2.2
Outer Solar System exploration