2010 Annual Science Report
 University of Wisconsin
Reporting | SEP 2009 – AUG 2010
University of Wisconsin
Reporting | SEP 2009 – AUG 2010
Project 3B: Do Iron-Rich Carbonates From Banded Iron Formations Record Ancient Seawater?
Project Summary
Carbonates are ubiquitous in the geologic record over Earth’s history, and their chemical and isotopic compositions have been key to discussions on the compositions of the ancient oceans, as well as the evolution of life. Moreover, the abundance of Fe-carbonates, common in banded iron formations (BIFs), and the Archean sedimentary rock record in general, has led many workers to use such carbonates as a proxy for surface conditions and to provide insights into seawater chemistry of the ancient Earth. For carbonates to yield information about ancient ocean compositions, however, they must be demonstrated to have been a direct precipitate from ocean water and not subsequently modified. One approach to test if ancient carbonates record seawater is through studies of the isotopic compositions of elements that usually reflect seawater compositions in carbonate minerals.
Project Progress
Archean carbonate minerals are often assumed to be direct precipitates of seawater, therefore acting as proxies to infer conditions of the ancient earth. More specifically, banded iron formation (BIF) carbonates record some of the largest excursions yet observed in C, O, and Fe isotope compositions for marine sedimentary rocks (Figure 1), and the origin of these variations have been highly debated. Large excursions towards highly negative δ13C values have been interpreted to reflect an ocean stratified in C isotope compositions for inorganic carbon (Beukes and Klein, 1990; Klein, 2005; Winter and Knauth, 1992), or, alternatively, microbial oxidation of organic matter during authigenic mineral formation (Becker and Clayton, 1972; Baur et al., 1985; Fischer et al., 2009). Low-δ18O values in Precambrian carbonates and cherts have been interpreted to record high seawater temperatures (e.g., Knauth, 2005), or to reflect seawater that had negative δ18O values (e.g., Kasting et al., 2006). The origin of variations in Fe isotope compositions of Precambrian carbonates and shales has been debated, and has included interpretations that the isotopic compositions reflect changing ocean δ56Fe values (Rouxel et al., 2005; von Blanckenburg et al., 2008), or that the δ56Fe values represent diagenetic processes that record microbial dissimilatory iron reduction (DIR) (Yamaguchi et al., 2005; Beukes and Gutzmer, 2008; Johnson et al., 2008 a, b).
Figure 1. Temporal variations in carbon and iron isotope compositions for marine sedimentary rocks of Archean and Proterozoic age. Green horizontal band marks range in δ56Fe values for several hundred analyses of low-C, -S clastic sedimentary rocks. Vertical gray band marks time of major negative excursion in δ13C values for kerogen. Data sources from Johnson et al. (2008b) and Shields and Veizer (2002).
Two studies have been completed on this project, the first of which involved Fe, C, and O isotope compositions (Heimann et al., 2010) of Fe-carbonates from the 2.5 Ga Kuruman Iron Formation and underlying platform carbonates (calcite/dolomite) from the Gamohaan Formation, Transvaal Craton, South Africa. The second study focused on the Rb-Sr isotope compositions (Ludois et al., submitted) of the same material previously analyzed for Fe, C, and O isotope compositions, as well as Nd isotope and REE variations of interbedded shales. The Kuruman IF is correlative with the Hamersley Basin BIFs of NW Australia, but were subjected to lower grades of metamorphism, and therefore more likely to record unmodified isotope compositions in the carbonate minerals. Previous isotopic studies of these carbonates demonstrated large Fe isotope variability (δ56Fe= +1 to -1‰), low δ13C values (-20 to -1‰), and low δ18O values of ~21‰ in Fe-rich carbonates, relative to compositions expected for precipitation from seawater. Testing the validity of seawater proxies such as BIF carbonate minerals provides important tests of proposals that the ocean was stratified in δ13C values, had high ocean temperatures, or that atmospheric CO2 was high. In addition, these results provide insight into the cause of large excursions in δ56Fe values in the Neoarchean and Paleoproterozoic, which has been subject to great debate. Based on the C, O, and Fe isotope compositions, Heimann et al. (2010) inferred that the Fe carbonates in the Kuruman BIF were not in isotopic equilibrium with seawater when they formed, but instead reflect Fe carbonates that formed in the sedimentary pile prior to lithification, where dissimilatory iron reduction (DIR) mobilized isotopically light aqueous Fe, leaving the residual material isotopically heavy. This study provides important evidence for DIR as a metabolic pathway involved in BIF formation in the Neoarchean, and does not support the model proposed by Rouxel et al., (2005) that large Fe isotope excursions reflect changing ocean δ56Fe values.
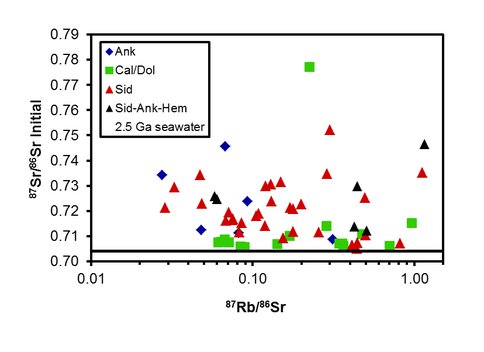
Our second study (Ludois et al., submitted) involved Rb-Sr isotope determinations on the same material previously analyzed for C, O, and Fe isotope composition by Heimann et al., (2010). Strontium is an important tracer of past ocean chemistry because Sr is isotopically homogenous in the oceans at any point in time, reflecting its long residence time, and, importantly, there is no significant fractionation during mineral precipitation, unlike stable isotope systems such as C, O, and Fe. Our results show that most Fe-carbonates have extremely radiogenic measured and initial 87Sr/86Sr ratios that plot far from the Sr isotope composition of Neoarchean seawater (87Sr/86Sr ~ 0.702-0.705) (Figure 2). The low 87Rb/86Sr ratios for most of the carbonates analyzed results in little age correction to the measured 87Sr/86Sr ratios. More importantly, there is no correlation between initial 87Sr/86Sr ratios and 87Rb/86Sr ratios, precluding physical clay (high Rb) contamination as an explanation for the radiogenic Sr isotope compositions. The origin of the high 87Sr/86Sr ratios in Fe-rich carbonate is likely a result of incorporation of radiogenic Sr derived from dispersed clay particles through ion-exchange in the soft sediment during authigenic mineral formation, prior to lithification. Such a process also explains the low δ13C and δ18O values of these Fe carbonates, as well as their wide range in δ56Fe values, over small scales (Figure 3).
Figure 2. 87Rb/86Sr vs. initial 87Sr/86Sr ratios of carbonate minerals from the Kuruman Iron Formation (Ank, Sid, Sid-Ank-Hem) and underlying platform carbonates (Cal/Dol). The black line at 87Sr/86Sr = 0.704 represents seawater composition at 2.5 Ga. Mineral abbreviations are siderite (Sid), ankerite (Ank), hematite-bearing siderite (Sid-Ank-Hem), calcite/dolomite (Cal/Dol). The very low 87Rb/86Sr ratios indicate that no significant siliciclastic or clay mineral component exists in the samples analyzed, and so cannot be invoked to explain the highly radiogenic Sr isotope compositions. Note log scale for Rb/Sr ratios.
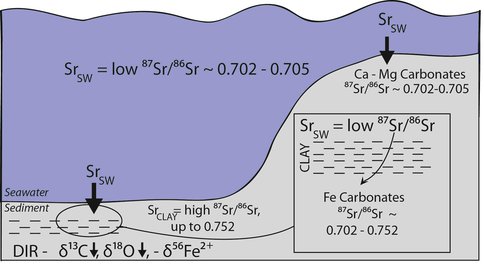
Figure 3. Formation model for BIF Fe-carbonates and underlying platform carbonates (Ca-Mg carbonates) in a non-stratified ocean, where Fe-carbonates (siderite/ankerite) formed in a deep marine environment by DIR, and Ca-Mg carbonates precipitated directly from seawater in shallow marine environments. Note that the range 87Sr/86Sr ratios indicated (0.702-0.705) encompasses the possible range in seawater in the Neoarchean and Paleoproterozoic, but is not meant to imply stratification. DIR produces aqueous Fe2+ with negative δ56Fe, low δ18O, and negative δ13C. Interaction between authigenic fluids in a sedimentary pile and surrounding clay minerals result in radiogenic 87Sr/86Sr incorporated into the authigenic fluids that later precipitate the BIF carbonates.
Combining the data from the studies provides strong evidence iron-rich carbonates cannot be assumed to be direct precipitates of seawater. The fact that the isotopic compositions of C, O, Fe, and Sr are decoupled indicate that these elements do not follow the same pathways during carbonate formation, and suggest that multiple chemical and isotopic proxies are needed to infer a seawater origin of carbonates. For example, REE contents of carbonates are a commonly used approach for inferring ancient seawater chemistry, but the results of this work demonstrates that this cannot be assumed. These results demonstrate that multiple isotopic systems, including those not directly cycled by life, provide important constraints on interpreting isotopic biosignatures in ancient rocks.
Publications
-
Heimann, A., Johnson, C. M., Beard, B. L., Valley, J. W., Roden, E. E., Spicuzza, M. J., & Beukes, N. J. (2010). Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in ~2.5Ga marine environments. Earth and Planetary Science Letters, 294(1-2), 8–18. doi:10.1016/j.epsl.2010.02.015
-
Johnson, C. M., Beard, B. L., Klein, C., Beukes, N. J., & Roden, E. E. (2008). Iron isotopes constrain biologic and abiologic processes in banded iron formation genesis. Geochimica et Cosmochimica Acta, 72(1), 151–169. doi:10.1016/j.gca.2007.10.013
- Johnson, C.M., Beard, B.L. & Roden, E.E. (2008b). The iron isotope fingerprints of redox and biogeochemical cycling in modern and ancient Earth: Annual. Rev. Earth Planet. Sci, 36: 457-493.
- Ludois, J.M., Johnson, C.M., Beard, B.L., Roden, E.E., Beukes, N.J. & Heimann, A. (2010, Submitted). Strontium isotopes in Banded Iron Formation carbonates demonstrate disequilibrium with ancient seawater.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Co-Investigator
Nicolas Beukes
Collaborator
Eric Roden
Collaborator
Mike Spicuzza
Collaborator
John Valley
Collaborator
Adriana Heimann
Postdoc
Jim Ludois
Graduate Student
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 4.3
Effects of extraterrestrial events upon the biosphere
Objective 5.2
Co-evolution of microbial communities
Objective 7.1
Biosignatures to be sought in Solar System materials