2010 Annual Science Report
 Pennsylvania State University
Reporting | SEP 2009 – AUG 2010
Pennsylvania State University
Reporting | SEP 2009 – AUG 2010
Biosignatures in Ancient Rocks
Project Summary
This team of geologists, geochemists, paleontologists and biologists seeks signs of early life in ancient rocks from Earth. Working mostly on that part of Earth history before the advent of skeletons and other preservable hard parts in organisms, our group focuses on geochemical traces of life and their activities. We also investigate how life has influenced, and has been influenced by changes in the surface environment, including the establishment of an oxygen-rich environment and the initiation of extreme climate states including global glaciations. For this we use a combination of observations from modern analogous environments, studies of ancient rocks, and numerical modeling.
Project Progress
Reinterpreting the sulfur MIF record—J. Kasting and J. Lyons
The goal of this project is to gain a better understanding of what can be learned about past atmospheric compositin by examining the record of sulfur mass-independent fractionation (MIF) in ancient rocks. In pursuit of this goal, Co-I Kasting and Mark Claire (an NAI postdoc) have developed a photochemical model that is similar in many respects to that of Pavlov and Kasting (2002). Those authors tracked only two sulfur isotopes: the main one, 32S, and a “mass-independent” one, S*. Furthermore, the initial fractionation process in their model was arbitrary because the cross sections of the SO2 isotopologues had not yet been measured. Our new model tracks three isotopes: 32S, 33S, and 34S. Mass-independent fractionation, or MIF, is measured by the deviation of the middle isotope, termed Δ33S, from the mass fractionation line defined by 32S and 34S. Our new model includes measured photolysis cross sections for the three corresponding SO2 isotopologues from the work of Danielache et al. (2008). These cross sections are relatively low resolution, so they do not include the detailed rotational fine structure of the various vibrational transitions. With Co-I Lyons, we hope eventually to obtain higher resolution spectra that will include the rotational fine structure.
Project 2 is the high-resolution measurement of SO2 isotopologue cross sections at Imperial College with the colleagues named above. The goal of the work is to measure isotopic SO2 spectra that can be used for accurate calculation of S-MIF effects during SO2 photolysis. This is necessary for Archean atmospheric models of sulfur isotope processes. After another round of measurements on the FTS (Fourier transform spectrometer) at Imperial College in July 2010, we have well characterized our noise level to be about 6-10 %. This is too high to address the S-MIF problem. The source of the noise is the D2 lamp. We are switching the FTS to another beam geometry in which the D2 lamp is monitored directly. This will greatly reduce the noise level. We hope to have these measurements done December 2010 – January 2011.
Analysis of our (noisy) cross sections is nevertheless useful. We searched for the wavelength dependence seen by Danielache et al. (2008) in their low-resolution spectra, and interpreted by Ueno et al. (2009) as evidence for very high Archean OCS levels. After making a constant (i.e., wavelength independent) baseline shift in the 33SO2 cross sections, we found good agreement between a model simulation and laboratory SO2 photolysis experiments of Pen and Clayton (2008). We then used these cross sections to search for the wavelength dependence seen by Danielache et al., but we do not see it. When we add OCS to our model atmosphere with the Imperial College cross sections, we do not get a change in sign of Δ33S for SO produced from SO2 photolysis. These results do not support the high OCS model suggested by Ueno et al.
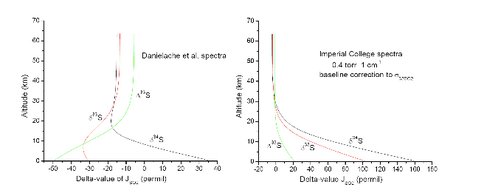
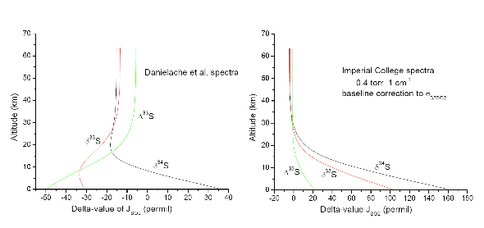
Figure 1. (left) Model calculations using the low-resolution spectra of Danielache et al. (2008). Here, JSO2 is the photodissociation rate coefficient. Line-type self-shielding is clearly not present (δ33S and δ34S should be the same sign), and is not expected given the lack of rotational detail in the spectra. (right) Model calculations using the moderate-resolution 1 cm-1 spectra from the Imperial College FTS. Line-type self-shielding is clearly present (δ33S and δ34S increase with depth). (Lyons et al., unpub.)
We are not the first researchers to use the Danielache et al. data in a model. Ueno et al. (2009) created a 0-D box model based on these cross sections. They predicted that one could reproduce the observed isotopic distribution in rocks—high Δ33S in sulfides and low Δ33S in sulfates—by shielding the lower atmosphere with high concentrations of carbonyl sulfide, OCS. The reason is that OCS, like ozone, has an absorption cross section that increases with wavelength in the critical 190-220 nm region where SO2 photolyzes. We, however, do not get these same results, probably because our model has vertical resolution, whereas Ueno’s model had none. Instead, we get the results shown in Fig. 2.
Figure 2. Diagrams illustrating preliminary results from the photochemical model being developed by Claire and Kasting. Left panel shows the vertical distribution of Δ33S values in different species. Note that sulfate aerosols have positive Δ33S values, and S8 aerosols have negative ones. Right panel shows the effect of OCS shielding on the average Δ33S values of aerosols removed at the surface. Adding OCS increases the spread between sulfate and S8 aerosols but does not change their sign. The right-hand scale shows the (presumably biogenic) OCS flux that would be needed to sustain a given OCS mixing ratio. By comparison, the current OCS flux is 3.6×109 mol/yr, or about 1.3×107 molecules cm-2s-1. So, the OCS flux needed to provide significant UV shielding is orders of magnitude higher than the present flux.
So, in short, we do not believe that the high COS concentrations predicted by Ueno et al. are correct. We would have published this conclusion already had not another paper appeared just recently that inserts a new variable into the system. Wolf and Toon (2010) showed that, if the organic haze particles produced from methane photolysis are assumed to have a fractal structure (consistent with haze particles on Titan), they can provide much better UV shielding than do spherical haze particles. We are currently adding fractal organic haze to our photochemical model to see whether this can change the sign of the Δ33S outputs.
We will also compare our results with those from another recent paper by Halevy et al. (2010). Like us, Halevy et al. used the Danielache et al. (2008) SO2 isotopologue cross sections. They presented a detailed biogeochemical model of how the sulfur MIF signal might be transmitted through the ocean and into sediments. Their atmospheric model, however, was extremely simple. It consisted of single, parameterized reaction
3 SO2 + hν → 2 SO3 + S (1)
The SO3 formed from this reaction was assumed to go directly into sulfate, after combining with H2O, whereas S was assumed to form solid elemental sulfur, S8. Their model predicts that the sulfur MIF signal should depend strongly on the H2S/SO2 ratio in volcanic gases, and hence on mantle oxidation state. By contrast, the Pavlov and Kasting (2002) model was relatively insensitive to this ratio because of rapid interconversions between sulfur species within the atmosphere. The Halevy et al. model is also insensitive to the total sulfur outgassing rate, whereas models with explicit atmospheric chemistry (Pavlov and Kasting, 2002; Ono et al., 2003) predict a strong dependence on this rate because of the nonlinear chemistry of S8 formation. Our present study will be the first to include the measured SO2 isotopologue absorption cross sections in a complete 1-D atmospheric model, and so we expect to be able to improve on these previous studies. The goal of this work is to use the observed sulfur MIF record to place quantitative constraints on atmospheric composition during the Archean eon.
Research Activities of the Hedges Group
Hedges continued his research on the refinement of the timescale of life, with emphasis on relating the origin of different groups of organisms to chemical and geological biomarkers. From a family-level treatment in 2009, his lab has expanded this scope to the species level, and is developing new methods for analysis of large sequence data sets. A smartphone application was developed and released for use with the lab’s TimeTree database.
The NAI research of Blair Hedges mainly concerns the refinement of the tree of life scaled to geologic time (i.e., the timetree of life). This information helps to relate chemical and geological biomarkers in the rock record to organisms that may have produced those biomarkers. In turn this provides a better understanding of biosphere evolution, and identifying possibly universal mechanisms and pathways applicable to life on other planets. In early 2009 the Hedges lab produced 12 publications bearing on this goal, including an edited book covering all of life down to the family level (S. B. Hedges & S. Kumar, The Timetree of Life, Oxford U Press). Since that project, research in the Hedges Lab has expanded, and now focuses on microbial evolution to the species level, which include thousands of prokaryote and eukaryote species (nearing completion). It was necessary to develop new methods to conduct this work, because existing programs are not capable of handling data sets of this size. New methods in turn require testing with computer simulations (e.g., Battistuzzi et al., 2010). In parallel to this empirical research we have continued development of a web database and resource, TimeTree (www.timetree.org), now holding divergence data from nearly 1000 published studies. in 2010 we also released a smartphone resource for this database, initially for Apple iPhone and related products. It contains a detailed geological timescale using standard colors and allows users to visualize all molecular data points in this reference scale, and explore more detailed information for each data point. In 2010, Hedges also directed, for the sixth season, the Astrobiology Summer Program (NSF-REU Site), which funds 10 undergraduates from universities and colleges across the U.S. to come to Penn State and astrobiology research with PSARC members.
Research Activities of Kump Group
The Kump Group’s focus has shifted during 2009-2010 to field work. We conducted a field campaign during the summer of 2010 collecting samples from Mesoproterozoic rocks in China to evaluate the “Canfield Ocean” hypothesis, that the Mesoproterozoic deep ocean was not only anoxic, but euxinic (had free hydrogen sulfide in the water column). The Chinese rocks are particularly interesting because they contain the expected black, pyrite-rich shales, but also green and red shales as well (Fig. 3). Work this fall (2010) has focused on developing the techniques to characterize the redox state of these rocks. We will be collaborating with Tim Lyons (UC Riverside, ASU NAI team) to develop a basin-wide perspective from these rocks, which span nearly 500 million years.
Figure 3. Red, green and black interbedded shales from the Chuanlingguo Formation, China (ca. 1700 Ma).
We also continue to pursue our work at Green Lake, NY State as an analog for the Canfield Ocean. Our first paper was published this year (Zerkle et al., 2010) and another is in review at Geobiology (Meyer et al., in review). We held an NAI co-sponsored field workshop at Green Lake this fall, with several NAI team members in attendance. The meeting focused on biosignatures of anaerobic phototrophic ecosystems in modern and ancient environments. It was attended by 52 researchers and graduate students.
Work also continues on the Late Permian mass extinction, with analyses and field work performed in conjunction with visiting graduate student Genming Luo. Several papers are in the works, including one paper on coupling of C and S cycles, that was just accepted into EPSL (Luo et al., in press).
Finally, our work on Paleozoic C and S cycling and links to anoxia has come to fruition with a paper in press in Nature (Gill et al., in press 2010).
*Research Activities of Ohmoto Group
during the period August 2009 – September 2010*
Reported by Hiroshi Ohmoto (Co-PI)
Group Members:
Hiroshi Ohmoto (Co-PI)
Dr. Yumiko Watanabe (Research Associate)
Ian Johnson (Ph.D. candidate)
Jamie Brainard (Graduate student)
Hiroshi Hamasaki (Graduate student)
Andrew Chorney (Undergraduate student, senior)
Thomas Rushton (Undergraduate student, senior)
Dennis Walizer (Research Technologist)
Collaborating scientists:
Prof. Jenn Macalady (Co-PI), Penn State University
Prof. Francis Albaréde, Ecole Normale Supérieure, Lyon, France
Prof. Janne Blichert-Toft, Ecole Normale Supérieure, Lyon, France
Dr. David Fernández Remolar, Centro de Astrobiologia, Spain
Dr. Ricardo Amils Pilbernat, Centro de Astrobiologia, Spain
Dr. Victor Casado Lafuente, Centro de Astrobiologia, Spain
Dr. Cesar Menor-Salvan, Centro de Astrobiologia, Spain
Dr. Fernando Tornos, Institute Geológico y Minero de España, Spain
Dr. Michael Doepel, Sipa Resources Ltd., Perth, Australia
Dr. Carl Brauhart, Sipa Resources Ltd., Perth, Australia
Dr. Arthur Hickman, Geological Survey of Western Australia
Dr. Andrew Fitzpatrick, CSIRO, Perth, Australia
Prof. Kosei Yamaguchi, Toho University, Japan
Prof. Clark Johnson, Univ. of Wisconsin, Madison
Prof. James Farquhar, Univ. of Maryland
Summary of Research Activities and New Discoveries
During this reporting period, we have focused on the following topics: (1) Geochemical cycles of redox-sensitive elements (especially O, S, C, Fe, Mo, U, W, and Cr) through the mantle – crust – ocean – atmosphere system of the early Earth; (2) Anomalous fractionation of sulfur isotopes; (3) Biogeochemistry of the Rio Tinto system; (4) Roles of methane hydrates in changing the atmospheric and oceanic chemistry. Following are brief summaries of our activities, new findings, and future directions related to each topic. Important conclusions from our investigations are highlighted in blue.
I. Geochemical cycles of redox-sensitive elements through the mantle – crust – ocean – atmosphere system of the early Earth
1.1. Investigations on trace element geochemistry of Archean submarine hydrothermal systems
(A). 3.46 Ga submarine basalts and jasper in ABDP #1 core
Molybdenum (Mo) is an essential bioelement for all Eukarya and Bacteria, and tungsten (W) is essential for nearly all Archea. An important question in Astrobiology has been “When did the oceans become enriched in Mo and W?” Concentrations of O2, S (SO42- and H2S), C (CO2 and HCO3-), Fe, Mo, U, V, Cr, Ce and many other redox-sensitive elements in oceans depend largely on the atmospheric chemistry (pO2 and pCO2), the composition and size of the continental crust, and the extent of seawater-basalt interactions (i.e., submarine hydrothermal processes) on mid ocean ridges.
Consequently, the mineralogy and chemistry of submarine basalts that were altered by submarine hydrothermal fluids and deep ocean water may be used to put constraints on the concentrations of redox-sensitive elements in the oceans, and also on the atmospheric chemistry. Through detailed investigations on 3.46 Ga submarine basalts and chert/jasper beds in ABDP #1 drill core, we have recently recognized that the mineralogy (especially the abundance of hematite) and chemistry (especially the contents of Li, Fe3+, U, Mo, W, V, Cr, and Ce) of these rocks are very similar to those of altered basalts from modern mid ocean ridges. These findings suggest that, already at ~3.5 Ga ago, (i) the deep ocean waters, as well as the surface waters and the atmosphere, were fully oxygenated, (ii) the geochemical cycles of many redox-sensitive elements were essentially the same as today, and (iii) Mo, W, and other bioessential elements were already available for a variety of organisms to develop. We have published an initial report in Nature Geosciences (Hoashi et al., 2009), presented progress reports in scientific meetings (Ohmoto et al., 2009, 2010), and we are now preparing extended reports of our findings for publication in major journals.
(B). 3.2 Ga submarine hydrothermal systems in the Panorama district, W.A.
We (Ohmoto, Brainard, Chorney and Rushton) carried out a three-week fieldwork (July-August, 2010) in the Pilbara district, Western Australia. Major objectives of this fieldwork were to study the geology of the Panorama district which host several volcanogenic massive sulfide deposits (VMS deposits) and Algoma-type banded iron formations (BIFs), and to collect the volcanic rocks associated with them in many drill cores. The collected drill core samples will be studied, mostly as a Ph.D. thesis research of Jamie Brainard and as a senior thesis research of Andrew Chorney, for the mineralogy and geochemistry to constrain the redox geochemistry of the oceans and atmosphere 3.2 Ga ago.
1.2. Investigations on Archean marine ecosystems
(A). 3.46 Ga Marble Bar Chert/Jasper Formation, Pilbara, W.A.
From investigations on organic geochemistry (C/N/S, δ13C, and δ15N of kerogen; δ13C of carbonates) and inorganic geochemistry of a ~160 m thick core section of the Marble Bar Chert/Jasper Formation in ABDP #1, we have come to suggest the existence of a diverse microbial communities in a 3.46 Ga sea: cyanobacteria in the photic zone, chemolithoheterotrophic methanogens in unconsolidated sediments, and chemolithoautotrophic methanogens in the vicinity of hydrothermal vents. Yumiko Watanabe have presented a part of the results at several scientific meetings (Watanabe et al., 2009, 2010), and will present a more complete report at the AGU meeting in December 2010.
(B). 2.7 Ga Black Shales in Kidd Creek Area, Ontario, Camada
From investigations on organic geochemistry of black shales from Kidd Creek, Watanabe suggests the existence of diverse microbial communities in an Archea sea, much like those in the 3.46 Marble Bar area. She has also recognized the enrichments of Mo in these shale samples, which show similar Mo/Corg ratios as those in the 2.5 Ga McRae Shale. If the Mo/C ratios can be correlated with the atmospheric pO2, as suggested by Anbar et al. (2008), our data would indicate a “Whiff of oxygen” at 2.7 Ga.
(C). 2.76 Ga Black Shales in ABDP #6 core
Black shales were not previously recognized in surface outcrops of the 2.76 Ga Mt. Roe Basalt Formation. However, we have found several thick layers of pyrite-rich black shales in ABDP #6 core. Tom Rushton has begun investigations on the organic and inorganic geochemistry of these black shales as his senior thesis research project.
1.3. Investigations on Archean paleosols
(A). 3.4 Ga lateritic paleosols in the North Pole Dome district, W.A.
Soils are formed by interactions of rocks with rainwater and soil organisms. Thus, mineralogy and geochemistry of paleosols may be used to constraint the atmospheric chemistry (pO2 and pCO2) and the nature of organisms on/in soils. Since our first discovery of “probable” paleosols on the oldest (~3.4 Ga) land surface in the North Pole Dome area in 2005, we have conducted: (a) 2-3 week fieldworks every year; (b) geophysical surveys (magnetic susceptibility, conductivity, and resistivity) during the summer of 2009; and© mineralogical and geochemical investigations of more than 100 rock specimens from the alteration zone. These investigations have been carried out mostly by Ian Johnson as parts of his Senior-, Master’s-, and Ph.D. thesis projects. We have found that the mineralogy and geochemistry of the 3.46 Ga paleosols, including the abundances of hematite, pyrophyllite and kaolinite, the absence of carbonates, and the behaviors of many redox-sensitive elements (Fe, Mn, U, Mo, Cr, etc), are essentially the same as those in modern soils formed in tropical regions. These data suggest that at 3.4 Ga ago, (i) the atmospheric pO2 was essentially the same level as today and (ii) subaerial biosphere was extensively developed.
(B). 3.4 Ga paleosols in the Eastern Pilbara district, W.A.
Since 2008 we have been investigating the distributions of the 3.4 Ga paleosols in the Pilbara district. So far we have discovered a 50-100 m-thick alteration zone underneath the 3.4 Ga unconformity in more than 20 locations in an area >100km x 200 km area, suggesting that the spatial extent of the 3.4 Ga soil formation was as large (or possibly larger) as the 2.2 Ga Hekpoort paleosols in South Africa.
II. Anomalous Isotopic Fractionation of Sulfur (AIF-S)
2.1. Experimental investigations
(A). AIF-S during thermochemical sulfate reduction by solid organic compounds
Watanabe et al. (2009) published a paper in Science, reporting a discovery that many of products (H2S, polysulfides) of reactions among solid organic matter (amino acids), solid sulfate, and water at 150-200°C possess distinct, but variable AIF-S signatures. We have further suggested that the AIF-S signatures in some Archean sedimentary rocks were created by reactions between immature organic matter (kerogen) and sulfate-rich pore water under the influence of submarine hydrothermal fluids during the diagenesis of sediments, rather than through the UV photolysis of volcanic SO2 gas in an O2-poor atmosphere. Watanabe is conducting similar experiments using remnants of cultured cyanobacteria, and organic matter extracted from the Green River Formation.
(B). AIF-S during reactions between SO2 and activated carbon (or anthracite)
To test the validity of a theoretical prediction by us (Lasaga et al., 2008) that chemisorption of S-bearing gasepus (or aqueous) species on solid surfaces may cause AIF-S, and also to understand the details of processes involved in the generation of AIF-S signatures in Watanabe’s thermochemical sulfate experiments, Hiroshi Hamasaki has been conducting series of experiments to investigate multiple sulfur isotope effects during adsorption and reduction of SO2 on activated carbon and coal (anthracite) at 200 and 250°C. This work will constitute a part of his MS thesis. He has found that the chemisorption-reduction of SO2 produced dihnificant fractionations up to ~4 ‰) in δ34S, and small but definite AIF-S signatures (Δ33S ≈ 0.3‰) in the reaction products (S2- and S0 on solid carbon compounds; SO2 residual). He will report the results of his study at the AGU meeting in December 2010.
2.2. Investigations on natural samples
(A). ABDP core samples
We have reported in Nature (Ohmoto et al., 2006) that sulfide minerals in the 2.76 Ga lake sediments of ABDP #2 drill hole and in the 2.92 Ga carbon-poor marine shales of ABDP #5 hole do not show AIF-S signatures. Subsequently, Watanabe analyzed multiple sulfur isotopic compositions of sulfides and sulfates in other ABDP cores (#1, #2, #6). She has also analyzed many samples of the 2.7 Ga Kidd Creek shales, compiled all the published data (>1000 data points) of multiple sulfur isotopic compositions on natural samples, and examined the relationships between the AIF-S characteristics and their geologic settings. We have recognized several types of correlations between the AIF-S signatures and depositional environments. We have concluded that the existence of such correlations are difficult to explain by the popular models linking AIF-S to atmospheric reactions, but they can be better explained by various combinations of chemisorption isotope effects, Rayleigh distillation isotope effects, and dissolution/reprecipitation of pyrite during diagenesis sediments. Frequent occurrences of AIF-S signatures in pre-2.5 Ga sedimentary rocks can be linked to the dominance of igneous activity in oceanic environments (rather than on land) where the three essential conditions for thermochemical sulfate reduction (the accumulation of large amounts of reactive organic matter, sulfate-rich seawater, and large-scale submarine hydrothermal activity) existed. Watanabe will present the results of investigations at the AGU meeting in December 2010.
(B). Sulfates and sulfides in 3.2 Ga volcanogenic massive sulfide deposits in the Panorama area, W.A.
Previous researchers have obtained AIF-S signatures on bedded bodies and massive veins of barite of ~3.46 Ga in age. These data are likely to represent the sulfur isotopic compositions of evaporated seawater in shallow basins that were located in margins of larger (semi)closed basins. Thermochemical sulfate reduction in the deeper parts of the sedimentary basins may have created the AIF-S signatures on the residual seawater SO42-. In order to determine the multiple sulfur isotopic ratios of SO42- in open oceans during the Archean, Andrew Chorney has begun sulfur isotope analyses of disseminated barite and pyrite in 3.2 Ga volcanogenic massive sulfide deposits in the Panorama area as a part of his senior thesis research.
III. Biogeochemistry of the Rio Tinto system
An outcome of collaborative research between Ohmoto and researchers at Centro de Astrobiologia in Spain (CAB), which begun in 2008, was the establishment of a formal collaborative program between PSARC and CAB in January 2010. The goal of the research collaboration is to increase our understanding of the origins, evolution, and distribution of life on Earth and other planets of the Solar system. We have been approaching this goal by answering the following specific questions: (1) What are the connections between the microbiology and geochemistry of the modern and ancient Rio Tinto systems (including surface and subsurface systems), which have been considered as an analogue for sulfuric environments on Mars?; (2) What types of paleontological and geochemical signatures of organisms in the Rio Tinto system may be used for the exploration of life (extinct and extant) on Mars?; (3) What was the nature of volcanic and hydrothermal activity on early Mars?; (4) What were the thermal and chemical conditions of the atmosphere and oceans on early Mars and Earth?; (5) How did the environments on early Mars and Earth influence the origin of life on each planet?; (6) How did the emergence and evolution of various organisms influence the environment (temperature and chemistry of the atmosphere and oceans) on Archean and Proterozoic Earth?; (8) What types of biosignatures in ancient terrestrial rocks may be useful for the exploration of life on other planets?
A major field expedition was carried out in May, 2010 by a group from PSARC (Ohmoto, Macalady, Watanabe, six graduate students (Jamie Brainard, Colin Carney, Hiroshi Hamasaki, Rebecca McCauley, Christen Miller, Rames Ramirez, and two undergraduate students (Danielle Gruen, Daniel Mills) together with a large group of researchers from CAB. We measured the various physical, chemical and biological characteristics of waters of the Peña de Hierro Lake and at many other locations of the Rio Tinto system in the field, and also on the collected water samples in the laboratories. During the four-month period preceding the field trip (Jan.-April), students registered for a 2-credit course (Astrobiology 570 Field Course) and prepared for the fieldwork by reading the pertinent literature and also practicing various laboratory techniques. After the fieldwork, the students have been continuously working on biological and chemical analyses of the Rio Tinto water samples. Through these investigations we were able to develop quantitative models for the evolution of S-Fe geochemistry of water in pyrite-rich environments and its influence on the microbial ecology. We are preparing manuscripts for publication.
IV. Roles of Methane Hydrates in Changing the Atmospheric and Oceanic Chemistry
Jamie Brainard and Ohmoto have been developing a new theory that quantitatively links the pulses of CO2- and CH4 increases in the atmosphere, the development of some large euxinic seas, and some extinction events in the geologic history to the scavenge of dissolved gases (O2, CO2, N2, etc.) in ocean water by the rising methane bubbles from the methane hydrate layer on ocean floors. Destruction of some methane hydrates can be linked to a drop in the global sea level and/or to an increase in bottom water temperature, which, due to deep-water circulation, typically occurs several hundred years after an increase in the surface temperature. Brainard will present this model at the AGU meeting in December 2010.
Publications
-
Jiang, S., Bralower, T. J., Patzkowsky, M. E., Kump, L. R., & Schueth, J. D. (2010). Geographic controls on nannoplankton extinction across the Cretaceous/Palaeogene boundary. Nature Geosci, 3(4), 280–285. doi:10.1038/ngeo775
-
Kump, L., Bralower, T., & Ridgwell, A. (2009). Ocean Acidification in Deep Time. Oceanography, 22(4), 94–107. doi:10.5670/oceanog.2009.100
-
Luo, G., Kump, L. R., Wang, Y., Tong, J., Arthur, M. A., Yang, H., … Xie, S. (2010). Isotopic evidence for an anomalously low oceanic sulfate concentration following end-Permian mass extinction. Earth and Planetary Science Letters, 300(1-2), 101–111. doi:10.1016/j.epsl.2010.09.041
-
Otake, T., Wesolowski, D. J., Anovitz, L. M., Allard, L. F., & Ohmoto, H. (2010). Mechanisms of iron oxide transformations in hydrothermal systems. Geochimica et Cosmochimica Acta, 74(21), 6141–6156. doi:10.1016/j.gca.2010.07.024
-
Saltzman, M. R., Young, S. A., Kump, L. R., Gill, B. C., Lyons, T. W., & Runnegar, B. (2011). Pulse of atmospheric oxygen during the late Cambrian. Proceedings of the National Academy of Sciences, 108(10), 3876–3881. doi:10.1073/pnas.1011836108
-
Young, S. A., Saltzman, M. R., Foland, K. A., Linder, J. S., & Kump, L. R. (2009). A major drop in seawater 87Sr/86Sr during the Middle Ordovician (Darriwilian): Links to volcanism and climate?. Geology, 37(10), 951–954. doi:10.1130/g30152a.1
-
Zerkle, A. L., Kamyshny, A., Kump, L. R., Farquhar, J., Oduro, H., & Arthur, M. A. (2010). Sulfur cycling in a stratified euxinic lake with moderately high sulfate: Constraints from quadruple S isotopes. Geochimica et Cosmochimica Acta, 74(17), 4953–4970. doi:10.1016/j.gca.2010.06.015
-
Zugger, M. E., Kasting, J. F., Williams, D. M., Kane, T. J., & Philbrick, C. R. (2010). LIGHT SCATTERING FROM EXOPLANET OCEANS AND ATMOSPHERES. The Astrophysical Journal, 723(2), 1168–1179. doi:10.1088/0004-637x/723/2/1168
- Gill, B., Lyons, T., Young, S., Kump, L., Knoll, A. & Saltzman, M.R. (2010, In Press). Did deep ocean anoxia stifle early 1 animal evolution during the later Cambrian? Nature.
- Hoashi, M., Watanabe, Y. & Ohmoto, H. (2009). Primary hematite formation in an oxygenated deep sea 3.46 billion years ago. 19th Goldschmidt conference, Davos, Switzerland. Geochim. Cosmochim. Acta, 73(13S): A536.
- Johnson, C.M., Yamaguchi, K.E., Poulson, S.R., Ohmoto, H. & Beard, B.L. (2009). Fe, S, and C isotopes record great microbial diversity in the Neoarchean. 19th Goldschmidt conference, Davos, Switzerland. Geochim. Cosmochim. Acta, 73(13S): A600.
- Johnson, I., Watanabe, Y., Stewart, B. & And Ohmoto, H. (2010). Evidence for terrestrial life and an O2-rich atmosphere in the oldest (~3.4 Ga) paleosol in the east Pilbara craton, Western Australia. 6th Astrobiology Sci. Conference. League City, TX.
- Johnson, I., Watanabe, Y., Stewart, B. & Ohmoto, H. (2009). Earth’s oldest (~3.4 Ga) lateritic paleosol in the Pilbara craton, Western Australia. 19th Goldschmidt conference, Davos, Switzerland. Geochim. Cosmochim. Acta, 73(13S): A601.
- Meyer, K.M., Kump, L.R., MacAlady, J., Schaperdoth, I. & Freeman, K. (In Review). Benthic production of a putative planktonic biomarker. Geobiology.
- Ohmoto, H. (2009). Redox evolution of volcanic gas through geologic time. 19th Goldschmidt Conference, Davos, Switzerland. Geochim. Cosmochim. Acta, 73(13S): A965.
- Ohmoto, H. (2010, In Press). Dioxygen. In: Henderson, J.C. & et al. (Eds.). Encyclopedia of Astrobiology.
- Ohmoto, H., Bevacqua, D.C. & Watanabe, Y. (2010). Hydrothermal alteration chemistry of Archean submarine volcanic rocks: evidence for a fully oxygenated atmosphere-ocean system since ~3.5 Ga. 6th Astrobiology Sci. Conference. League City, TX.
- Ohmoto, H., Bevacqua, D.C., Johnson, I. & Watanabe, Y. (2010). Geochemical cycles of Fe, Mo, U, Cu, Cr, REEs, and S during the period 3.5-3.2 Ga ago. Geochim. Cosmochim. Acta, 74(12S): A774.
- Otake, T., Lasaga, A.C., Watanabe, Y. & Ohmoto, H. (2009). Theoretical investigations of anomalous fractionation of sulfur isotopes during a surface reaction. AGU Fall Meeting, San Francisco, CA. EOS, 90(52): V11A-1932.
- Watanabe, Y. & Ohmoto, H. (2009). Why anomalous S isotope signatures disappeared at 2.4 Ga ago? 19th Goldschmidt Conference, Davos, Switzerland. Geochim. Cosmochim. Acta, 73(13S): A1420.
- Watanabe, Y. & Ohmoto, H. (2010). Connection between depositional environments and multiple sulfur isotope ratios of sulfides and sulfates in Archean sedimentary rocks. 6th Astrobiology Sci. Conference. League City, TX.
- Watanabe, Y., Hoashi, M. & Ohmoto, H. (2010). Evidence for the presence of cyanobacteria and thermophylic methanogens in a 3.46 Ga sea, Western Australia [Goldschmidt conference]. Geochim. Cosmochim. Acta, 74(12S): A1116.
- Yamaguchi, K.E., Kato, Y., Nakamura, K., Suzuki, K., Watanabe, Y., Nedachi, M. & Ohmoto, H. (2009). REE+Y geochemistry of the 3.46 Ga Marble Bar Chert recovered by the Archea Biosphere Drilling Project. 19th Goldschmidt Conference, Davos, Switzerland. Geochim. Cosmochim. Acta, 73(13S): A1469.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Fabia Battistuzzi
Collaborator
James Farquhar
Collaborator
Christopher Junium
Collaborator
Sudhir Kumar
Collaborator
Timothy White
Collaborator
Aubrey Zerkle
Collaborator
James Fulton
Postdoc
Yumiko Watanabe
Research Staff
Ying Cui
Doctoral Student
Hiroshi Hamasaki
Doctoral Student
Matthew Heinicke
Doctoral Student
Lev Horodyskyj
Doctoral Student
Genming Luo
Doctoral Student
Stamatina Hunter
Graduate Student
Ian Johnson
Graduate Student
Nathan Barber
Undergraduate Student
Jamie Brainard
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.1
Earth's early biosphere.
Objective 4.2
Production of complex life.
Objective 4.3
Effects of extraterrestrial events upon the biosphere
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems