2010 Annual Science Report
 Montana State University
Reporting | SEP 2009 – AUG 2010
Montana State University
Reporting | SEP 2009 – AUG 2010
Surface Chemistry on Iron-Sulfur Minerals
Project Summary
Progress has been made in defining competitive abiotic pathways for reducing nitrogen compounds to ammonia from nitrogen oxides relative to the dinitrogen. Using pyrite mineral surfaces and freshly precipitated Fe-S particles, we showed that under hydrothermal conditions nitrite (NO2-), nitrate (NO3-), as well as nitric oxide (NO) can be converted to ammonia to comparable yields than starting from dinitrogen (N2). Formation of ammonia or ammonium ion in aqueous solution is considered as an essential step toward creating amino acids that are key building blocks of life.
Project Progress
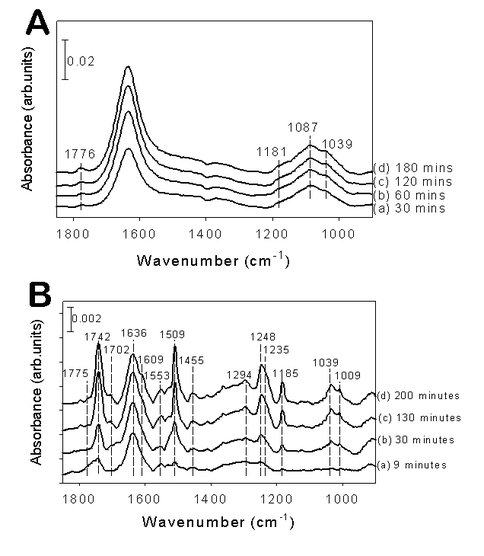
A possibly catalytic process was discovered on pyrite surfaces involving nitrite (NO2-) in aqueous media that can be considered an extension of earlier work in the Schoonen laboratory. In the earlier study a redox conversion of freshly precipitated FeS showed improved ammonia formation in water from dissolved hydrogen sulfide (H2S(g)) and dinitrogen (N2(g)) at hydrothermal temperatures (T ~ 120°C) relative to the background ammonia signal in the absence of FeS. Our current studies suggest that a novel pathway may be operative where ammonia formation is driven by a disproportionation reaction of nitrite (NO2-) to nitrate (NO3-) and nitric oxide (NO), and the latter species becomes further reduced in concomitant steps to ammonia. A systematic experimental effort was completed to map the parameter space of temperature, iron-sulfur particle loading, and presence of dinitrogen/nitrite/nitrate substrates. Surface complexes were indentified using attenuated total reflection Fourier Transform infrared spectrophotometer (ATR-FTIR), which eliminates the intense water absorbances found in traditional FTIR measurements and allows for detection of signals for species adsorbed on the surface in low concentrations.
Room temperature (~22 °C) experiments using FeS particles and either NO3- or NO2- substrates did not show any ammonia formation. However, increasing the temperature toward hydrothermal conditions (~ 70 °C) showed ammonia formation (85 M concentration within a day), but only when NO2- is used as the substrate. Nitrate did not show any reactivity. Kinetic measurements revealed that the amount of ammonia formed is dependent on the initial nitrite concentration. In contrast to FeS, a more modest (~10 M per day), but unambiguous ammonia formation was detected on pyrite (FeS2) for both nitrate and nitrite at elevated temperatures. This reaction could further be enhanced by elevating the reaction temperature into the true hydrothermal regime of 120 °C.
Comparison of the ATR-FTIR spectral signatures of nitrite adsorption on pyrite surface at 22 ºC (A) and 70 ºC (B) temperatures as a function of time (a-d as noted in figures)
Publications
-
Singireddy, S., Gordon, A. D., Smirnov, A., Vance, M. A., Schoonen, M. A. A., Szilagyi, R. K., & Strongin, D. R. (2012). Reduction of Nitrite and Nitrate to Ammonium on Pyrite. Orig Life Evol Biosph, 42(4), 275–294. doi:10.1007/s11084-012-9271-8
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Martin Schoonen
Project Investigator
Daniel Strongin
Project Investigator
Robert Szilagyi
Co-Investigator
Alexander Smirnov
Postdoc
Michael Vance
Postdoc
David Gardenghi
Graduate Student
Alex Gordon
Graduate Student
Soujanya Singireddy
Graduate Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems