2010 Annual Science Report
 Montana State University
Reporting | SEP 2009 – AUG 2010
Montana State University
Reporting | SEP 2009 – AUG 2010
Rationalized Chemical Surface Modifications
Project Summary
Using biological examples such as nitrogen fixation by nitrogenase, hydrogen evolution and uptake by hydrogenases, and reversible CO/CO2 conversion by CO dehydrogenase, we began to study the effect of heterometal (Mo, V, Ni) substitution in iron-sulfur minerals and particles. We have successfully bound molybdenum sulfide on pyrite mineral surfaces and exploring the synthetic feasibility of doping Ni into freshly precipitated FeS particles. Preliminary reactivity studies indicated higher yields in formation of ammonia from nitrogen oxides at hydrothermal conditions relative to the pure iron-sulfur systems.
Project Progress
Classical Fe-S systems with symmetrical sulfur coordination are capable of mediating rapid electron-transfer processes (2Fe and 4Fe ferredoxins and 4Fe high potential iron proteins) with practically no chemical reactivity. However, upon perturbation of either the cluster composition (nitrogenase with Mo, V; hydrogenase with Ni; carbon monoxide dehydrogenase with Ni) or the coordination environment (oxygen and nitrogen ligation in SAM radical enzymes) drastically alters the electronic structure of Fe-S systems with relatively small structural perturbations and define a wide variety of chemical reactivity from small molecule activation (N2, H2, CO/CO2), to atom transfer (H, S), to even the complicated C-C bond cleavage reaction. Relevantly, these heterometal insertion or perturbations in the interaction of the Fe-S clusters and their organic environment allow for the above reactions to take place under catalytic conditions. Thus these systems provide a very strong rational for the extension of our investigations of Fe-S surfaces in stoichiometric and catalytic reactions.
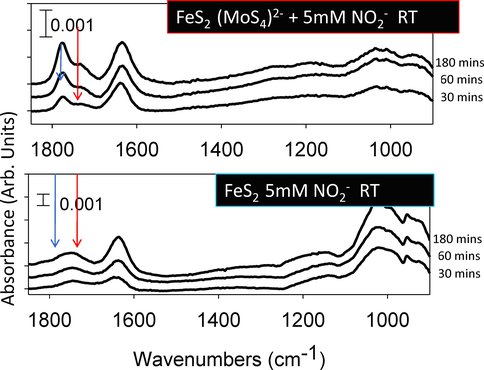
We have started to chemically modify various iron-sulfur surfaces, including but not limited to pyrite with thio and oxomolybdates (MoS42-, MoO42-) that sorb as surface complexes with a cubane structure. These heterometal substituted cubanes are the most straightforward mimics of metalloenzyme active sites, such as the MoFe3S4 cubane of the nitrogenase FeMo-cofactor. We are exploring similar chemistry to be able to place Ni2+ cations with a controlled coordination environment. The samples are being prepared and characterizations will be carried out using X-ray spectroscopic measurements in November. The reactivity studies will include the investigation of their catalytic activity in breaking N,N single (hydrazine), double (dimethyldiazine), and ultimately triple bonds (dinitrogen) under hydrothermal conditions using reducing sources such as NO2- disproportionation, presence of reduced transition metals (Cu(I)), cyanide as a mild reducing agent, etc. These investigations will be an important step towards bridging the gap between bio- and geocatalysts.
Preliminary results showing the increased number of surface species of molybdenum sulfide modified pyrite surface upon nitrite binding already at room temperature
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Martin Schoonen
Project Investigator
Daniel Strongin
Project Investigator
Robert Szilagyi
Co-Investigator
Alexander Smirnov
Postdoc
Michael Vance
Postdoc
David Gardenghi
Doctoral Student
Alex Gordon
Doctoral Student
Soujanya Singireddy
Doctoral Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems