2010 Annual Science Report
 NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2009 – AUG 2010
NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | SEP 2009 – AUG 2010
Path to Flight
Project Summary
Our technology investigation, a Path to Flight for astrobiology, utilizes instrumentation built with non-NAI funding to carry out three science investigations namely habitability, survivability and detectability of life. The search for life requires instruments and techniques that can detect biosignatures from orbit and in-situ under harsh conditions. Advancing this capacity is the focus of our Technology Investigation.
Project Progress
Investigation 4- Path to Flight Investigation Progress
The (Field Instrumentation and) Path to Flight investigation’s purpose is to enable in-situ measurements of organics and biological material with field instrumentation that have high potential for future flight instrumentation. The preceding three Investigations (Habitability, Survivability and Detectability) provide a variety of measurable goals that are used to modify or “tune” instrumentation that can be placed in the field. In addition the members involved with Investigation provide new measurement capabilities that have been developed with the specific goal of life-detection and organic detection using both non-contact/non-destructive means and ingestion based methods. The developments under this investigation (Inv 4) incorporate state-of-the-art laboratory instruments and next generation in-situ instrumentation that have been developed under programs that include NASA as well as NSF and DOD. These include mass-spectrometers, gas analyzers, and fluorescence/Raman spectrometry instruments.
The Path to Flight investigation has three (3) primary Objectives, all of which have been progressing in year 2 . Below are the objectives and a list of accomplished tasks.
OBJECTIVE1: Chemistry at Hydrothermal Vents (Laboratory simulator and field environment)
- Verify measurements of chemicals formed and consumed in laboratory simulations of alkaline hydrothermal vents and modify those measurement instruments into ones capable of shallow depth measurements at a natural alkaline vent (near Iceland).
OBJECTIVE2: Survivability and Presence in Ice
- Perform biochemical and organism measurements inside deep polar ice cores archived at the National Ice Core Lab (Colorado) and/or Montana State University.
- Develop/Re-tune 2D mapping stage for the Deep UV fluorescence/Raman instruments
OBJECTIVE3: Ground Truth and Observability
- Modify existing set of field instruments to enable their use in major field campaigns around Barrow, Alaska.
The following are some additional details associated to the accomplished tasks and next steps in the FY11.
Year 2 Progress:
1. Hydrothermal Vent Chemistry:
The JPL development and modification of the laboratory based, high pressure, low temperature, JPL Hydrothermal Vent, is described in Mielke et al (2010) and for analysis of laboratory grown hydrothermal chimney formation (detailed in Inv.1). The developments in FY 2010 focused on modification of the instruments to enable analysis of effluent (liquid and gas) from the JPL Hydrothermal Vent Simulator (HVS) and mineralogical analysis of the hydrothermal chimney formations.
Gas produced by the JPL HVS was analyzed using a slightly modified version of TLS (Tunable Laser Spectrometer) that is a part of the SAM GC/MS instrument on the upcoming Mars rover, MSL. The modification made is possible to detect dimethyl sulfoxide (DMS) and methyl mercaptan (CH3SH). Liquid and dissolved gas analysis of the vent fluid is still under development. For dissolved gases, a gc/differential mobility spectrometer (GC/DMS) is using a novel method for gas extraction from a liquid. The design when complete will allow extraction of dissolved gases from salt water. For liquid analysis for organics that may be produced by the vent (such as acetate) we are using both Raman and resonance-Raman spectroscopy; the latter utilizes a deep UV source at 248 nm that enhances the Raman scattering effect up to 6 orders of magnitude. The deep UV Raman module comes from an ASTID development of a collaborating company (Photon Systems) and is currently being tested on low concentrations of acetate. The goal FY11 is to incorporate this to directly to a modified version of the HV and monitor the fluid in-situ.
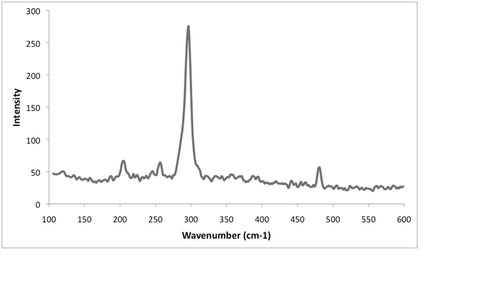
In addition to the JPL Hydrothermal Vent Simulator, mineralogical analysis was performed on lab-grown chimneys. The details of the chimney formation are provided in Inv1. To summarize biological processes from the modern to the earliest indications of life utilize ferrodoxins and iron-sulfides protein clusters in electron transport. It is possible that simpler versions of these structures could be formed abiotically and act as organic traps for simple organics produced by serpentization reactions where they would react at FeS “membranes” utilizing the disequilibrium to catalyze reactions. One iron-sulfide mineral of particular interest is the formation of makinawite which may be used to catalyze organic reactions. However, another iron-sulfide, pyrite, inhibits this catalysis. To determine the iron-sulfide being created, a sample of the iron-sulfide based chimney was placed into a nitrogen purged quartz cylinder to prevent alteration by oxidation. Using the new JPL multi-wavelength Raman imaging spectrometer, we were able detect nano-crystalline makinawite (Fig 4.1). This analysis began with summer student (now ASU graduate student) Kirt Robinson and will continue in the coming year with graduate student Lauren White.
Fig. 4.1. Nano-crystalline Makinawite from laboratory hydrothermal chimney vent formations. There is no indication of pyrite in this sample.
2. Biological Material in Ice Cores:
The proposed effort under this task was to use the ice core repository at the National Ice Core Labs (NICL) in Boulder, CO as samples to scan with multiwavelength deep UV Raman and fluorescence spectrometers and subsample for analysis by GC/MS, GC/DMS, HPLC for organics, and FPLC for proteins. However, late in 2009, the USGS indicated the support for NICL was potentially going to end. During this time, however, CO-I J. Priscu was finishing the construction of an ice core facility in Montana State University. This provided a suitable option for NICL; and in many ways assisted collaboration with MSU.
As stated in FY 2009, we were in progress of modifying X-Y 1m x 0.5m scanning stage. This was completed in FY10 with results on related work (described later) being published in Nov 2010 1. Although this was originally developed to scan cores at NICL, it is likely that this system will be used in Barrow, Alaska to scan cores using the deep UV instrument, soon after they are extracted.
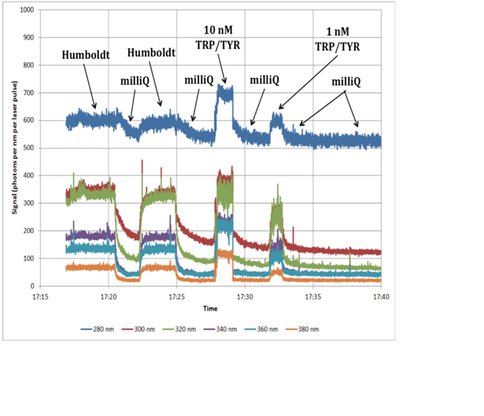
Given the changes at NICL, Inv. 4 support ice-core analysis of biological materials by adding supporting funding to co-I J. Priscu in acquistion of a new Targeted Ultraviolet Biological Sensor (TUBS) from our industry collaborators, Photon Systems. This would not only support the NAI goals with analysis support from co-I R. Bhartia, the new instrument will assist in analysis of ice-cores from the Antarctic and Greenland Ice Sheets under an NSF project (PI: J. Priscu). The instrument incorporates a 224 nm excitation laser and 6 emission wavelengths (280, 300, 320, 340, 360, 380 nm) to measure a continuous stream of melted ice. The outflow from the TUBS is then fed to a Model 900 Port, Turbo TOC Analyzer manufactured by GE Analytical to provide the total organic carbon concentration. Initial tests of the TUBS are shown in Figure 4.2. This work is continuing into Year 3.
Fig. 4.2. TUBS Time trace of emission for samples from the Humboldt ice core (Greenland), milliQ water and various concentrations of the amino acids tryptophan (TRP) and tyrosine (TYR). (Graph courtesy of Photon Systems).The TUBS emission spectra clearly show that all emission wavelengths respond to the ice core sample and standards and decrease when milliQ water is pumped through the system. Importantly, the ratios of the different emission spectra change between the ice core sample, the amino acid standards and milliQ water, indicating that each has different qualities.
3.Instrumentation Efforts for Field Use:
Barrow, Alaska Campaigns
Much of the field instrumentation will be used in Barrow, Alaska in April of FY11. The developments include a tip/tilt deep UV mapping instrument, an ice-core scanning system described earlier, and an electrospray Miniature Mass Spectrometer (Figure 4.3). Results from these instrument will be discussed in the next annual Report.
ANSMET Organic Contamination Analysis
During the last ANtarctic Search for METeorites (ANSMET), our collaborator, Marc Fries (Planetary Science Institute), fielded a deep UV native fluorescence instrument to detect organics present either through human or natural contamination (Figure 4.3). The benefit of this instrument is its 1-3 meter standoff capability and a high affinity to detect organics. The instrument did perform, however the UV from the sun was much greater than previously anticipated. Aside from providing new accurate UV radiation information, this affected the dynamic range of the instrument. However, organics were detected and analysis is presently underway.
Fig. 4.3. TUCBE instrument involved in ANSMET field work in Antarctica (FY2010), looking for possible PAH fluorescence signatures around undisturbed meteorites.
Deep Biosphere Microbial Assement Instrument
Investigation 4, with R. Bhartia, has also been collaborating with University of Southern California (PI: K. Edwards), Photon Systems, Inc. (Covina, CA), and Lamont Doherty (Columbia University, NY), in developing a wireline borehole instrument (DEBI-T) that utilizes deep ultra-violet (DUV; <250nm) fluorescence for label-free detection of microbes and organics on the walls of the borehole. In September/November 2011, DEBI-T will be deployed into Hole 395A, at North Pond as a part of the Integrated Ocean Drilling Project’s (IODP) Mid-Atlantic Ridge Microbiology Expedition (PI: Dr. Katrina Edwards – USC), which aims to elucidate the microbial communities inhabiting oceanic crust down to 600 m beneath the ocean floor (5000m below sea level). DEBI-T’s role during the cruise will be to provide real-time maps of the microbial and organic abundance and distribution on basalts as a function of depth, and to aid in more effective deployment of in-hole incubation and fluid sampling systems.
The ability to detect microbes in the natural environment has recently been shown in our recent publication (Bhartia et al App Environ. Microbiol. Nov 2010). In a similar manner, the DEBI-T uses a pulsed, compact, 224.3 nm DUV laser to elicit a fluorescence response that can be used to differentiate different groups of organic and biological materials (Bhartia et al Applied Spectroscopy 2010). This includes vegetative bacterial cells, bacterial spores, nitrogen and sulfur-bearing organic heterocycles and polycyclic aromatic hydrocarbons (PAHs). This capability will allow DEBI-T to assess microbial abundance as a function of depth with the hopes of distinguish microbial variation based on protein expression and heterocyclic compounds residing on the rock matrix.
To support this development, Investigation 4 will be supporting a new Caltech/JPL post-doc beginning in November 2010.
The Robotic Chemistry Analysis Laboratory (RCAL):
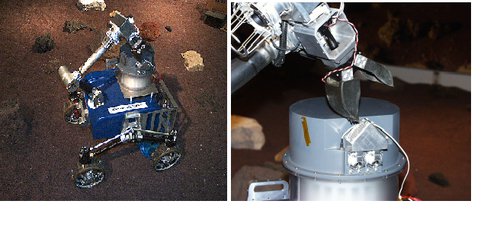
RCAL had been mounted on the SRR-2K rover nand all power and controls now come from the rover (Fig 4.4). Earlier trials had identified shortcomings in some of the hardware and many actuators have now been replaced by encoded motors to give more reliable and precise control, coupled with appropriate changes to the operations software.
Fig. 4.4. RCAL sample hand off is shown. On the left is the RCAL mounted on the SRR-2K rover. On the right, the arm has just delivered a soil sample to the hopper of the RCAL.
Over summer 2010, we started testing a new design of sensor, coated wire ion selective electrodes (Fig. 2). Initial tests showed good sensitivity and linearity but there were some problems with ion selectivity and a more electrodes is being fabricated.
Fig. 4.5. On the left is the previous Ion Selective Electrode (ISE) made by Orion Co. is shown. On the right is the coated Wire ISE made in the laboratory by our collaborator, Sam Kounaves, is shown. The previous design allowed co-mounting of five electrodes, but the form factor of the new design allows as many as ten electrodes in each probe.
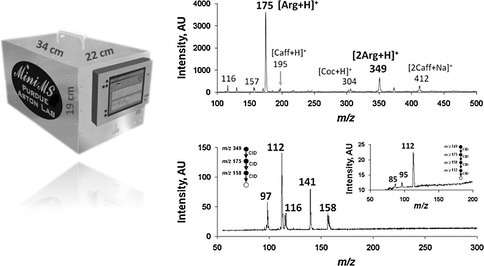
Miniature Field Portable Mass Spectrometer: As a part of investigation 4, we reported on the use of a small light-weight mass spectrometer (MS) for chemical analysis of organic material directly from solution or from the solid state with potential value in future planetary missions. The mass spectrometer used in the experiments reported here is handheld and controlled from a laptop computer through custom software. Detection and identification of small organic molecules, including some that might be prebiotics, was achieved using methods relevant to in situ and remote sensing applications. The miniature MS was equipped with a discontinuous atmospheric pressure interface (DAPI) and a home-built electrosonic spray ionization (ESSI) source. Aqueous solutions of molecules of interest were examined using the ESSI technique, while desorption electrospray ionization (DESI) was applied to examine solid samples. The system performance was characterized by direct analysis of analytes belonging to several compound classes including biotic and abiotic amino acids, purines, pyrimidines, nucleosides and peptides. Detection limits in the sub-ppm range for solutions were achieved with the atmospheric pressure sampling/ionization interface. Tandem mass spectrometry (MS2) was successfully applied to confirm trace detection of target compounds in mixtures. Multiple stage (MSn) analysis, where n = 3–5, was employed for molecular structure confirmation and to demonstrate the high chemical specificity as well as the sensitivity of the instrumentation. The use of improved versions of this type of mass spectrometer on exploration missions could provide detailed chemical information on organic materials in physical states currently difficult to access. The high sensitivity and specificity, combined with rapid detection and the absence of requirements for sample preparation are encouraging features of the instrumentation.
Fig. 4.6. Portable mass spectrometer, with potential value for planetary missions, was used to detect and identify amino acids, purines, pyrimidines, nucleosides and peptides at sub-part per million levels in solution using MS and MSn.
Publications
-
Bhartia, R., Salas, E. C., Hug, W. F., Reid, R. D., Lane, A. L., Edwards, K. J., & Nealson, K. H. (2010). Label-Free Bacterial Imaging with Deep-UV-Laser-Induced Native Fluorescence. Applied and Environmental Microbiology, 76(21), 7231–7237. doi:10.1128/aem.00943-10
-
Mielke, R. E., Russell, M. J., Wilson, P. R., McGlynn, S. E., Coleman, M., Kidd, R., & Kanik, I. (2010). Design, Fabrication, and Test of a Hydrothermal Reactor for Origin-of-Life Experiments. Astrobiology, 10(8), 799–810. doi:10.1089/ast.2009.0456
-
Sokol, E., Noll, R. J., Cooks, R. G., Beegle, L. W., Kim, H. I., & Kanik, I. (2011). Miniature mass spectrometer equipped with electrospray and desorption electrospray ionization for direct analysis of organics from solids and solutions. International Journal of Mass Spectrometry, 306(2-3), 187–195. doi:10.1016/j.ijms.2010.10.019
- Kanik, I., Beegle, L.W., Johnson, P.V., Hodyss, R. & Young, D.T. (2010, to be submitted). Mass spectrometers and other analytical separation devices for planetary applications: a critical review. Review of Scientific Instruments.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Max Coleman
Co-Investigator
Kevin Hand
Co-Investigator
Michael Russell
Co-Investigator
Steven Vance
Co-Investigator
Robert Cooks
Collaborator
Marc Fries
Collaborator
Randall Mielke
Research Staff
Ewa Sokol
Doctoral Student
Kirtland Robinson
Graduate Student
Lauren Spencer-White
Graduate Student
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 1.2
Indirect and direct astronomical observations of extrasolar habitable planets.
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems