2009 Annual Science Report
 University of Wisconsin
Reporting | JUL 2008 – AUG 2009
University of Wisconsin
Reporting | JUL 2008 – AUG 2009
The Role of Dissolved Sulfide in Controlling Carbonate Mineral Compositions
Project Summary
Role of microbes in dolomite formation is still under debate. It was proposed that sulfate-reducing bacteria (SRB) can results in dolomite precipitation. We investigated the role of dissolved sulfide (a product of SRB metabolism) in controlling carbonate mineral compositions and even dolomite crystallization at low temperatures. Our results show that very high-magnesium calcite (VHMC) and disordered dolomite can precipitate from aqueous sulfide-bearing solutions at low temperature.
Project Progress
Carbonate minerals with continuous compositional variation occur in a Martian meteorite (ALH 84001). Mechanisms for causing this are still under debate. We propose that sulfate-reducing bacteria may result in dolomite formation. We investigated the role of dissolved hydrogen sulfide in controlling precipitation of high-magnesian calcite and dolomite at low temperature. The origin of dolomite and high-Mg calcite remain unsolved problems in geochemistry that bear on the evolution of surface conditions on the early Earth and Mars. We found that both the concentration of the dissolved sulfide and temperature affect the amount of MgCO3 incorporation into carbonate crystals, such that very high-Mg calcite (VHMC) may be produced. It is proposed that dissolved HS-, a Lewis soft base, interacts with H+ in the water dipoles that bind to Mg2+ ions at VHMC or dolomite surfaces, which weakens chemical bonds between the water dipoles and surface Mg2+ ions. We suggest that this weakening lowers the kinetic energy barrier for the dehydration of surface Mg2+-water complexes, and therefore enhances Mg2+ incorporation into VHMC and dolomite. In contrast, dissolved sulfate has been considered to inhibit dolomite formation. However, our experiments show that VHMC and disordered dolomite can grow in solution almost saturated with gypsum only if aqueous sulfide is present, which emphasizes the significance of dissolved sulfide in dolomite formation.
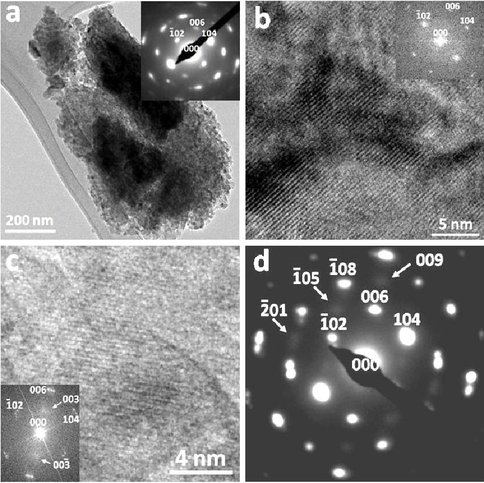
Ca-Mg ordering occurred after annealing the disordered dolomite at 300 °C for 140 hours (Fig. 1). This means, in natural environments, either very slow crystallization or further aging of disordered dolomite in aqueous sulfide-bearing pore water systems will eventually result in ordered or partially ordered dolomite during diagenesis.
Fig. 1: a: TEM image showing disordered dolomite nano-crystals (~ 50 mole% of MgCO3). Inset is a [010] zone-axis SAED pattern, which shows that there was no Ca-Mg ordering and these nano-crystals are not at random orientations, but with low-angle grain boundaries. b: HRTEM image of the disordered dolomite (~50 mole% of MgCO3) with (006) lattice fringes, no (003) superlattice fringes. Inset is a [010] zone-axis FFT pattern showing no super-lattice reflections. c: HRTEM image of the annealed dolomite showing (003) supperlattice fringes that characterizing Ca-Mg order. Inset is a [010] zone-axis FFT pattern showing weak super-lattice reflections. d: SAED pattern from the annealed dolomite. Arrows indicate weak super-lattice reflections like 003, 009, and 05 characterizing Ca-Mg order.
Based on our synthesized carbonate minerals in the calcite and disordered dolomite series, we have proposed a new approach for measuring composition in the calcite – disordered dolomite solid solution series based on XRD, because X-ray diffraction has been used widely in analyzing Ca-Mg carbonates. Compositions of biogenic and inorganic (Ca,Mg)CO3 crystals are often calculated by comparing measured d104 value with published empirical curves. However, the deficiency of these curves prevented their application to very high-Mg calcite and disordered dolomite. Based on synthesized high-Mg calcite and disordered dolomite, a new empirical curve between magnesian calcite d104 values and MgCO3 content in the calcite – disordered dolomite solid solution series is constructed. This new curve revealed the significant cell parameter changes accompanying the Mg-Ca cation disorder in dolomite, and it can help the characterization of the MgCO3 content of both natural and synthetic magnesian calcite and disordered dolomite, especially for the mineral mixtures which are not suitable for other analysis methods.
Publications
-
Xu, H. (2010). Synergistic Roles of Microorganisms in Mineral Precipitates Associated with Deep Sea Methane Seeps. Geomicrobiology: Molecular and Environmental Perspective, None, 325–346. doi:10.1007/978-90-481-9204-5_15
-
Zhang, F., Xu, H., Konishi, H., & Roden, E. E. (2010). A relationship between d104 value and composition in the calcite-disordered dolomite solid-solution series. American Mineralogist, 95(11-12), 1650–1656. doi:10.2138/am.2010.3414
- Zhang, F., Xu, H., Konishi, H. & Roden, E.E. (2009). A relationship between d-104 value and composition in the calcite – disordered dolomite solid solution series. Annual Meeting Geological Society of America. Portland, Oregon.
- Zhang, F., Xu, H., Konishi, H. & Roden, E.E. (2010, In Review). Sulfide-catalyzed nucleation and growth of very high-magnesian calcite and disordered dolomite, precursors to sedimentary dolomite. Earth and Planetary Science Letters.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Hiromi Konishi
Unspecified Role
Eric Roden
Unspecified Role
Fangfu Zhang
Unspecified Role
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems