2009 Annual Science Report
 University of Wisconsin
Reporting | JUL 2008 – AUG 2009
University of Wisconsin
Reporting | JUL 2008 – AUG 2009
Production of Mixed Cation Carbonates in Abiologic and Biologic Systems
Project Summary
Carbonate minerals commonly occur on Earth and they are found in extraterrestrial materials such as meteorites and interplanetary dust particles. The chemistry of carbonates provides clues about their formation and alteration of over time. For example, carbonate minerals that form inorganically have chemical compositions that are highly constrained by the environmental conditions under which they grow, however, it is now known that microorganisms can produce carbonates that deviate from these generally accepted patterns. As such, when carbonate minerals are placed in the appropriate environmental context, certain compositions may represent a biosignature for microbially mediated formation. The goal of this project is to develop a broader understanding of how carbonate minerals grow so that we may formulate explicit criteria for their origin based on their chemical and isotopic composition.
Project Progress
Year two accomplishments centered on inorganic free-drift experiments to synthesize carbonate minerals of “mixed cation” (Fe-Mg-Ca) content at 25°, 35°, and 45°C. These experiments may be broken down into two groups: 1) those using chloride salt reagents (32 exps), and 2) those using perchlorate salts (7 exps). Once mixed, the experimental solutions were stored in containers for one month, after which they were opened and the solution and solid run products were characterized by ICP-OES, titration. XRD, TGA, acid digestion, SEM and TEM.
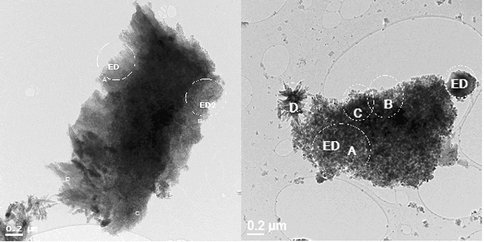
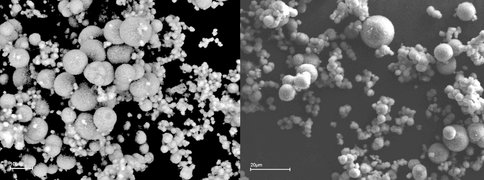
The experiments yielded siderite, calcian siderite and ankerite, with the texture, chemistry and mineralogy of the precipitates being dependent on the initial chemistry of the solution. SEM and TEM analysis revealed that all solids displayed a rhomohedral (Fig. 1a) or spheroidal habit, with some grains also forming nano-crystalline aggregates (Fig.1b).
In the absence of aqueous Ca, pokrovskite and ferric oxide precipitated from some solutions that were relatively high in Mg (50 to 100 mM) and low in Fe (0 to 2 mM). It is unclear if these phases are the result of aqueous oxidation processes or the oxidation of reactive solids during storage prior to analysis. Thermodynamic modeling (PHREEQC-2.15.0 and PHRQPITZ ) of the solution compositions support the experimental findings.
Noteworthy results include:1) ferroan dolomite and/or ankerite may be precipitated inorganically from solution at less than 45ºC, 2) the double carbonate (Ca,Fe)(CO3)2 was synthesized from solution at less than 45ºC, 3) ferroan dolomite and ankerite cannot be distinguished from Ca-or Mg-siderite by XRD analysis alone due to the similar crystal structures and competitive effects of Ca and Mg on unit cell parameters for dolomite, ankerite and siderite, and 4) a pre-existing solid (e.g., Ca-siderite) may facilitate the dehydration of aqueous Mg for incorporation into ferroan dolomite at temperatures (e.g., 25ºC) that were thought to be kinetically inhibited previously.
These results suggest that some unusual carbonate chemistries may form at relatively low temperature through heterogeneous reactions. Other phases still exist such as Mg-siderite, and pure dolomite and magnesite, that have yet to be grown inorganically at relatively low temperature (e.g., 25ºC). Because microbially mediated analogues are known to exist in nature, future research will specifically target formation mechanisms for these phases.
Figure 1a. Rhombohedral aggregates of Ca-siderite nano-particles from run S22. Goethite in right hand panel (at D).
Fig. 1b Spheroidal aggregates of nano-particles from run S25. Note varying size classes.
Publications
-
Romanek, C. S., Jiménez-López, C., Navarro, A. R., Sánchez-Román, M., Sahai, N., & Coleman, M. (2009). Inorganic synthesis of Fe–Ca–Mg carbonates at low temperature. Geochimica et Cosmochimica Acta, 73(18), 5361–5376. doi:10.1016/j.gca.2009.05.065
- Jimenez-Lopez, C., Romanek, C.S. & Bazylinski, D.A. (In Press). Magnetite as a Prokaryotic Biomarker. Journal of Geophysical Research-Biogeosciences.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Max Coleman
Collaborator
Nadine Kabengi
Collaborator
Nita Sahai
Collaborator
Huifang Xu
Collaborator
Monica Sanchez Roman
Postdoc
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials