2009 Annual Science Report
 University of Wisconsin
Reporting | JUL 2008 – AUG 2009
University of Wisconsin
Reporting | JUL 2008 – AUG 2009
Microbial Pyrite Oxidation in Nature and the Lab: Sulfate Mineral Biosignature Investigation
Project Summary
Deposits of minerals containing the chemical species sulfate, a constituent of ocean water, have been identified on the surface of Mars. Although there might be other methods for its formation, the process we are investigating is one that occurs on Earth and is the result of the metabolic activity of microbes, which oxidize the iron sulfide mineral pyrite (Fool’s Gold) to form sulfate. We have made the oxygen in water in a bacterial culture medium traceable by addition of a non-radioactive isotopic tracer and can thus differentiate oxygen from the atmosphere, used by biological processes, from oxygen in water, used by the non-biological processes. Our new data and approaches defy conventional wisdom, and offer a novel way for investigating the origin of sulfates on Mars by in situ instruments.
Project Progress
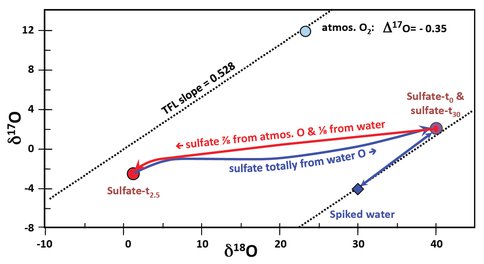
Extensive sulfate beds have been observed and detected remotely on Mars. The oxidation of pyrite (iron sulfide) by bacteria is one of the mechanisms by which sulfate is produced. We have made definitive measurements from our microbial experiments in the laboratory and have quantified the stable isotopic effects that differentiate microbial from abiotic processes. In our experiments we have cultured Acidithiobacillus ferrooxidans (Af) and analyzed the oxygen isotope composition of the sulfate it produces from oxidation of pyrite. The point that makes our experiments different from all previous ones is that we not only use water in the culture medium that is isotopically enriched 18O, but also analyze all three isotopes of oxygen to give δ17O and δ18O. We have successively grown cultures of Af in our isotopically enriched water. This microbe can oxidize both sulfur and iron but gains more energy from the former. Therefore, a new culture which is initiated by inoculating 1 ml. of a mature culture in 150 ml of a new sulfate-free one, starts growing by oxidizing the sulfide mainly using atmospheric oxygen, but leaves the iron in the Fe(II) form in solution. After a period of time, approximately 2.5 days in our experiments, the bacteria start to oxidize the Fe(II) in solution to produce Fe(III). However, Fe(III) oxidizes pyrite sulfide much more rapidly than the bacteria and at that stage they grow very rapidly by just oxidizing Fe(II) to Fe(III) to release yet more Fe(II). In this second stage of growth the sulfate is formed abiotically with oxygen from water as the oxidant. The isotopic values measured in our experiments are shown in Figure 1, which plots δ17O against δ18O for sulfate and the water spiked with H218O.
Figure 1. Triple oxygen isotope results for sulfate and spiked water in growth experiments using an enriched isotope culture medium. See text for an explanation.
Any isotopic fractionation process usually modifies the δ17O to about half the extent of the δ18O. This is the Terrestrial Fractionation Line (TFL) shown with a slope of 0.528. Differences of δ17O from that line are presented as Δ17O values. Our spiked water has 18O added and so has a large, negative Δ17O value. Atmospheric oxygen is characterized by a δ18O of +23‰ and a Δ17O of -0.35‰. The point marked, Sulfate-t0, is the value for a newly inoculated culture but still has a little sulfate from the previous growth. As the culture starts growing and producing sulfate the O isotope trajectory tracks towards the Sulfate-t2.5 point, the value after 2.5 days. In this initial period of growth, seven parts of the oxygen used for oxidation are atmospheric to one part from water. After this period, the chemical analyses show that Fe(II) oxidation has started and the sulfate is then produced by the abiotic process using just water oxygen. The major growth period produces much more sulfate tacking back to the original value (which, of course, had been produced by an identical process).
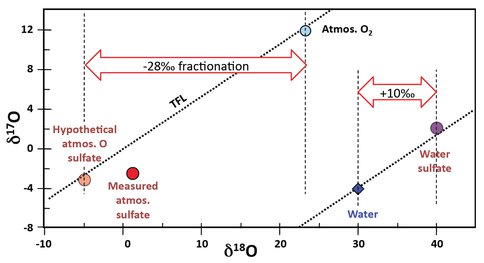
We have been able to make a preliminary interpretation of the data but are in the process of making the final replicate measurements and doing the mass balance calculations that will produce the final and most accurate values that characterize the two processes. The interpretation is shown in Figure 2.
Figure 2. Interpretation of triple oxygen isotope results to define the isotopic fractionations of sulfate oxygen formed by either microbial or inorganic processes. See text for an explanation.
The Sulfate-t2.5 point has almost all its sulfate produced by microbial oxidation using atmospheric oxygen and so, as for any other fractionation process, should lie on the TFL. The fact that it does not can be explained, because only seven eighths of its O comes from the atmosphere and the other eighth from water. If we assume that the isotopic value of the sulfate produced from water is similar to that of the abiotic process that dominates the second part of the microbial growth, then we can extrapolate by one seventh of the distance the mixing line of Sulfate-t0 (water-O sulfate) and along the Sulfate-t2.5 (atmosphere-O sulfate) to find the pure atmospheric-O sulfate value. Despite the approximation for the water-O sulfate value, the calculated point is very reassuring on the TFL for atmospheric oxygen.
Our initial estimates of the measured values for oxygen isotope fractionation from these two processes are: -28‰ for aerobic, atmospheric-O, biological fractionation and +10‰ for the abiotic process. These values are very different from the accepted wisdom of the most recent publications, which give values of approximately -10‰ and -3.5‰, respectively (Balci et al., 2007 and references therein).
The next steps needed to complete this work for publication are in hand. We are making the last sets of replicate analyses to confirm the (small) uncertainties in the data and will then make the final calculations.
Also, over the last year we have expended quite a lot of effort to grow Af and Leptospirrilum ferrooxidans, a bacterium that solely oxidizes Fe(II), at temperatures as low as 4°C. After some initial, apparent success, we have found that adaptation to both low temperature and a sulfate-free medium has resulted in strains that grow very slowly. We may just have to run very long duration experiments to capture the comparative data, which not only are more comparable to those on Mars, but also will allow us to understand better the basis for the measured fractionation factors.
This work on characterization of oxygen utilization pathways and oxidation kinetics will help to define specifications for new instruments for identifying mechanisms of formation of sulfates by in situ analyses on Mars.
Publications
- Brunner, B., Yu, J-., Mielke, R.E. & Coleman, M. (2008). Different chemical and isotopic signatures of pyrite oxidation by Acidithiobacillus ferrooxidans during lag and exponential growth phases. Earth Planet. Sci. Let, 270: 63-72.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Edward Young
Collaborator
Karen Zeigler
Collaborator
Randall Mielke
Research Staff
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 7.1
Biosignatures to be sought in Solar System materials