2009 Annual Science Report
 University of Wisconsin
Reporting | JUL 2008 – AUG 2009
University of Wisconsin
Reporting | JUL 2008 – AUG 2009
Iron Isotope Biosignatures: Laboratory Studies and Modern Environments
Project Summary
The isotopic fingerprints of biological carbon and sulfur cycling in modern and ancient marine environments is well established by research over several decades, but, until recently, potential iron isotope fingerprints of microbial iron cycling in the ancient Earth have not been pursued. Next to carbon, iron was probably the most important element cycled by early life, given the high abundance of iron in early Earth environments and the energy gains that may be obtained by microbes during iron redox changes. Our new laboratory studies moved away from simple systems to those more analogous to nature, and we demonstrated that iron isotope fractionations can be produced by biological cycling in complex systems. Moreover, in a field study, we isolated natural iron cycling microbes and demonstrated that the iron isotope fractionations produced by natural microbial ecosystems are the same as those produced by pure strains in the laboratory; these are key components to confidently applying Fe isotopes as a biosignature for ancient life.
Project Progress
There is a growing number of studies that interpret negative δ56Fe values for Fe(II)aq and sediments in natural systems to reflect dissimilatory iron reduction (DIR) (e.g., Bergquist and Boyle 2006; Severmann et al. 2006; Staubwasser et al. 2006; Fehr et al. 2008; Severmann et al. 2008; Homoky et al. 2009; Tangalos et al. 2009; Teutsch et al. 2009). These interpretations are based on the results of experimental investigations of relatively simple systems (e.g., Beard and Johnson 1999; Beard et al. 2003; Johnson et al. 2005; Crosby et al. 2007) that are actually poor analogs to natural environments. Nevertheless, a number of workers have applied these results to Precambrian marine sedimentary rocks, suggesting that negative δ56Fe values, particularly for Fe-rich rocks, most likely reflect ancient DIR (e.g., Yamaguchi et al. 2005; Archer and Vance 2006; Jenkyns et al. 2007; Johnson et al. 2008a; 2008b; Severmann et al. 2008); this interpretation is not universally accepted, however, where other workers have favored abiological processes for production of negative δ56Fe values in the ancient rock record (Rouxel et al. 2005; Anbar and Rouxel 2007). An important component to testing the application of Fe isotopes as a biosignature for Fe-based metabolisms on the early Earth or other planetary bodies such as Mar, lies in careful experimental work under controlled laboratory simulations of natural conditions.
In Year 2 we completed two studies of the Fe isotope fractionations produced by abiologic and abiologic Fe(II)aq-hematite interactions, with an emphasis on the effects of pH and dissolved silica, which have not been studied before. The pH of the Archean oceans is very difficult to constrain, and we sought to investigate the effects of neutral and mildly alkaline pH. In addition, dissolved Si was likely to be at amorphous silica solubility concentrations in the Archean and Proterozoic oceans because of the lack of silica-secreting organisms in the Precambrian. Under conditions of high pH and dissolved silica, the Fe isotope fractionations produced by DIR are decreased relative to those produced by DIR under neutral pH conditions (with or without dissolved silica; Fig. 1), although they remain of sufficient magnitude to be recorded in the rock record (Wu et al. 2009a). Our results obtained under abiological conditions of Fe(II)aq-hematite exchange confirm the proposal by Crosby et al. (2007) that the fundamental mechanism for producing Fe isotope fractionation during DIR of hematite lies in equilibrium isotopic exchange between Fe(II)aq and reactive Fe(III) on the oxide surface. Crosby et al. (2007) suggested that the role of bacteria was to catalyze isotopic exchange via electron pumping to the surface of iron oxides.
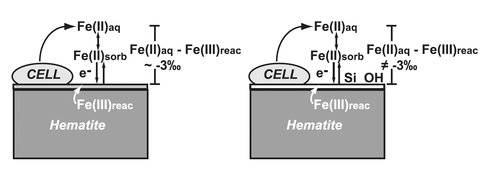
Figure 1. Schematic illustration of bacterial reduction of hematite. Part of the aqueous Fe(II)
(Fe(II)aq) produced sorbs to the hematite surface (Fe(II)sorb), and then undergoes electron transfer and Fe(II)–Fe(III) atom exchange, producing a reactive layer of Fe(III) at the oxide surface (Fe(III)reac) that has 56Fe/54Fe ratios which are higher than those of the initial hematite, balanced by Fe(II)aq that has 56Fe/54Fe ratios which are lower than the initial hematite. The left side shows overall isotopic fractionation between Fe(II)aq and Fe(III)reac (3 per mil), the expected fractionation where Fe(III)reac is hematite. The right side, however illustrates the changes observed in the presence of Si, and at high pH, where fractionation between Fe(II)aq and Fe(III)reac does not reflect that of the equilibrium Fe(II)aq-hematite fractionation, but are more modest, suggesting that the Fe(III)reac component is a phase other than hematite or that Fe bonding is significantly different than that in the Si-absent, circum-neutral pH experiments. From Wu et al. (2009a).
The new results obtained on abiologic systems (Wu et al., 2009b) demonstrate that isotopic exchange may occur in where Fe(II) and ferric Fe oxide are in close proximity, most likely requiring significant sorbed Fe(II). During processes such as abiologic oxidation of Fe(II)aq, continued isotopic exchange will have the result of decreasing apparent Fe isotope fractionations that are produced by Rayleigh processes, but indistinguishable from processes that reflect equilibrium fractionation between Fe(II)aq-hematite. Because, however, abiologic Fe(II)aq-hematite interactions are limited to exchange with the outermost layer of hematite (Fig. 1), the quantities of Fe(II)aq that may be isotopically shifted by this process is limited. During DIR, active reduction of the ferric Fe oxides (the terminal electron acceptor) continually exposes new oxide surfaces, and therefore significantly larger quantities of negative δ56Fe Fe(II)aq may be produced by DIR relative to abiologic exchange, even thought the mechanism of fractionation is the same. This conclusion is a modification of the Crosby et al. (2007) proposal, where we now envision the “biological pump” to be the process of exposing new surface layers of oxide that undergo Fe isotope exchange with ambient Fe(II)aq. It therefore remains likely, in our opinion, that the large inventory of Fe-rich, low-δ56Fe marine sedimentary rocks in the Archean and Proterozoic rock record may be confidently ascribed to DIR.
In Year 2, we also completed a combined field and experimental study of Fe isotope fractionations produced by DIR. Although the studies of modern marine sediments and lacustrine systems by Bergquist and Boyle (2006), Severmann et al. (2006; 2008), Staubwasser et al. (2006), Fehr et al. (2008), Homoky et al. (2009), and Teutsch et al. (2009) all call upon DIR to produce the observed Fe isotope compositions in pore fluids and sediments, none of these studies demonstrated, through microbiological approaches, that DIR was responsible for Fe cycling in the samples studied. Moreover, modern marine sediments are poor analogs for Archean marine conditions because the high sulfate levels of today’s oceans can exert a significant influence on Fe cycling through production of sulfide by bacterial sulfate reduction. Deposition of chemical sediments such as banded iron formations, a common lithology in the Precambrian, required very high Fe fluxes, and low sulfide, as compared to the fluxes in modern marine sediments (e.g., Klein 2005).
We addressed these issues through a combined field and laboratory study, including chemical, isotopic, and microbiological analyses of chemically precipitated sediments in the Spring Creek Arm of the Keswick Reservoir (SCAKR), downstream of the Iron Mountain acid mine drainage site in northern CA (Figure 2). Because these sediments record very high fluxes of Fe, relative to S, they are a unique analog to early diagenetic progenitors of banded iron formations in Archean and Proterozoic marine environments. A 16S rRNA gene sequence clone library from the SCAKR sediment contained sequences closely related (97% similarity) to known dissimilatory iron-reducing microbes (Geobacter and Geothrix). In addition, SKACR sediments were incubated in the laboratory; comparison of reactive Fe inventories and isotopic compositions of in situ sediment samples with the results of the incubation experiments produced identical results, providing, for the first time, a conclusive demonstration that DIR in natural systems are responsible for the observed Fe isotope fractionations that previously has only be inferred from simple laboratory experiments (Tangalos et al., 2009).
Figure 2. Aerial photo (upper middle) of SCAKR study site showing location of acid mine drainage derived, Fe(III) oxide-rich sediment (“Pile C”) from which cores (far left) were obtained for chemical, microbiological, and Fe stable isotopic analysis. TEM analysis showed that the sediment contains aggregates of nm-sized goethite (middle panels A and B) and ferrihydrite (middle panels C and D) crystallites. Incubation of the Fe(III) oxide-rich sediment under anaerobic conditions (lower right) led to microbial production of aqueous and solid-associated Fe(II) that had Fe isotopic composition similar to values measured in sediment core materials.
Publications
-
Fehr, M. A., Andersson, P. S., Hålenius, U., & Mörth, C-M. (2008). Iron isotope variations in Holocene sediments of the Gotland Deep, Baltic Sea. Geochimica et Cosmochimica Acta, 72(3), 807–826. doi:10.1016/j.gca.2007.11.033
-
Homoky, W. B., Severmann, S., Mills, R. A., Statham, P. J., & Fones, G. R. (2009). Pore-fluid Fe isotopes reflect the extent of benthic Fe redox recycling: Evidence from continental shelf and deep-sea sediments. Geology, 37(8), 751–754. doi:10.1130/g25731a.1
-
Johnson, C. M., Beard, B. L., Klein, C., Beukes, N. J., & Roden, E. E. (2008). Iron isotopes constrain biologic and abiologic processes in banded iron formation genesis. Geochimica et Cosmochimica Acta, 72(1), 151–169. doi:10.1016/j.gca.2007.10.013
-
Tangalos, G. E., Beard, B. L., Johnson, C. M., Alpers, C. N., Shelobolina, E. S., Xu, H., … Roden, E. E. (2010). Microbial production of isotopically light iron(II) in a modern chemically precipitated sediment and implications for isotopic variations in ancient rocks. Geobiology, 8(3), 197–208. doi:10.1111/j.1472-4669.2010.00237.x
-
Teutsch, N., Schmid, M., Müller, B., Halliday, A. N., Bürgmann, H., & Wehrli, B. (2009). Large iron isotope fractionation at the oxic–anoxic boundary in Lake Nyos. Earth and Planetary Science Letters, 285(1-2), 52–60. doi:10.1016/j.epsl.2009.05.044
-
Wu, L., Beard, B. L., Roden, E. E., & Johnson, C. M. (2009). Influence of pH and dissolved Si on Fe isotope fractionation during dissimilatory microbial reduction of hematite. Geochimica et Cosmochimica Acta, 73(19), 5584–5599. doi:10.1016/j.gca.2009.06.026
- Anbar, A.D. & Rouxel, O. (2007). Metal stable isotopes in paleoceanography. Annu Rev Earth Pl Sc, 35(1): 717-746.
- Archer, C. & Vance, D. (2006). Coupled Fe and S isotope evidence for Archean microbial Fe(III) and sulfate reduction. Geology, 34(3): 153-156.
- Beard, B.L. & Johnson, C.M. (1999). High precision iron isotope measurements of terrestrial and lunar materials. Geochim Cosmochim Acta, 63(11-12): 1653-1660.
- Beard, B.L., Johnson, C.M., Skulan, J.L., Nealson, K.H., Cox, L. & Sun, H. (2003). Application of Fe isotopes to tracing the geochemical and biological cycling of Fe. Chem Geol, 195(1-4): 87-117.
- Bergquist, B.A. & Boyle, E.A. (2006). Iron isotopes in the Amazon River system: Weathering and transport signatures. Earth Planet Sci Lett, 248(1-2): 54-68.
- Crosby, H.A., Roden, E.E., Johnson, C.M. & Beard, B.L. (2007). The mechanisms of iron isotope fractionation produced during dissimilatory Fe(III) reduction by Shewanella putrefaciens and Geobacter sulfurreducens. Geobiol, 5(2): 169-189.
- Jenkyns, H., Matthews, A., Tsikos, H. & Erel, Y. (2007). Nitrate reduction, sulfate reduction and sedimentary iron-isotope evolution during the Cenomanian-Turonian Anoxic Event. Paleoceanography, 22(PA3208).
- Johnson, C.M., Beard, B.L. & Roden, E.E. (2008). The iron isotope fingerprints of redox and biogeochemical cycling in modern and ancient Earth. Planetary Sciences, 36: 457-493.
- Johnson, C.M., Roden, E.E., Welch, S.A. & Beard, B.L. (2005). Experimental constraints on Fe isotope fractionation during magnetite and Fe carbonate formation coupled to dissimilatory hydrous ferric oxide reduction. Geochim Cosmochim Acta, 69(4): 963-993.
- Klein, C. (2005). Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origin. Am Mineral, 90(10): 1473-1499.
- Rouxel, O.J., Bekker, A. & Edwards, K.J. (2005). Iron isotope constraints on the Archean and Paleoproterozoic ocean redox state. Science, 307: 1088-1091.
- Severmann, S., Johnson, C.M., Beard, B.L. & McManus, J. (2006). The effect of early diagenesis on the Fe isotope compositions of porewaters and authigenic minerals in continental margin sediments. Geochim Cosmochim Acta, 70(8): 2006-2022.
- Severmann, S., Tw, L., Anbar, A., McManus, J. & Gordon, G. (2008). Modern iron isotope perspective on the benthic iron shuttle and the redox evolution of ancient oceans. Geology, 36(6): 487-490.
- Staubwasser, M., Von Blanckenburg, F. & Schoenberg, R. (2006). Iron isotopes in the early marine diagenetic iron cycle. Geology, 34(8): 629-632.
- Yamaguchi, K.E., Johnson, C.M., Beard, B.L. & Ohmoto, H. (2005). Biogeochemical cycling of iron in the Archean-Paleoproterozoic Earth: Constraints from iron isotope variations in sedimentary rocks from the Kaapvaal and Pilbara Cratons. Chem Geol, 218(1-2): 135-169.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Co-Investigator
Eric Roden
Co-Investigator
Charles Alpers
Collaborator
Christopher Kennedy
Collaborator
Huifang Xu
Collaborator
Lingling Wu
Postdoc
Hiromi Konishi
Research Staff
Evgenya Shelobolina
Research Staff
Heidi Crosby
Graduate Student
George Tangalos
Graduate Student
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 4.1
Earth's early biosphere.
Objective 5.2
Co-evolution of microbial communities
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems