2009 Annual Science Report
 Rensselaer Polytechnic Institute
Reporting | JUL 2008 – AUG 2009
Rensselaer Polytechnic Institute
Reporting | JUL 2008 – AUG 2009
Project 6: The Environment of the Early Earth
Project Summary
Our project entitled “Environment of the Early Earth” involves the development of capabilities that will allow scientists to obtain information about the conditions on early Earth (3.0 to 4.5 billion years ago) by performing chemical analyses of crystals (minerals) that have survived since that time. When they grow, minerals incorporate trace concentrations of ions and gaseous molecules from the local environment. We are conducting experiments to calibrate the uptake of these “impurities” that we hope will serve as indicators of temperature, moisture, oxidation state and atmosphere composition. To date, our focus has been mainly on zircon (ZrSiO4), but we have recently turned our attention to quartz as well.
Project Progress
Research at the Rensselaer (New York) Astrobiology Center under the theme 'Environment of Early Earth' was initiated on several fronts during FY09. The rationale behind these efforts is described in two specific sections of our 2008 NAI proposal: 7.2.2 Hydrous species as indicators of fluid pressure and composition and 7.2.3 solubility of atmospheric gas molecules in common rock-forming minerals. In addition, we undertook an unplanned experimental study of the incorporation of chemical species in quartz as potential indicators of the environment and conditions of quartz crystallization (and, ultimately, the nature of the early Archean crust).
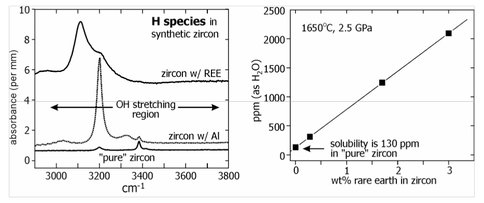
Hydrous species in zircon (ZrSiO4) as indicators of fluid pressure and composition. There are two primary objectives of this sub-project. The first is to obtain, through synthesis of zircons at high temperatures in the presence of H2O, a calibration of the identity, amount and diffusive mobility of hydrous species (H2O or OH−) incorporated into the nominally anhydrous ZrSiO4 structure. The second objective is to analyze the Hadean zircons from Western Australia’s Jack Hills for lattice-bound H species and evaluate the likelihood of this 'trapped water’ as a faithful record of the environment of crystallization on the early Earth. During the first 9 months of NAI funding we developed a technique for synthesis of zircons sufficiently large to analyze for dissolved H species using polarized-light Fourier transform infrared spectroscopy (FTIR). Our experiments confirm that zircons do indeed incorporate H species when grown in the presence of H2O at elevated pressure-temperature conditions (Fig. 1). It is clear, however, that the mechanism of H-species uptake can depend upon the availability and identity of trivalent cations in the system—in particular Al3+ and the trivalent rare earths (REE). This finding may complicate the direct application of our results to natural zircons, but we are nevertheless very encouraged by these early results.
Figure 1. Left: FTIR spectra of synthetic zircons crystallized from an aqueous fluid phase in the presence
of rare earth elements (REE) or aluminum, or as pure ZrSiO4. Note the marked differences in the positions and strength of the absorption peaks, which seems to indicate that the presence of 3+ cations (REE3+ or Al3+) dramatically increases the uptake of H species in the zircon (compare the area under the peaks for the “pure” case with those containing 3+ ions). The differences in the spectra of REE- and Al-bearing zircons suggest that OH− may be associated specifically with these impurities in the zircon structure. Right: The concentration of H species expresses as ppm H2O shows an excellent correlation with zircon REE content, further supporting the conclusion that OH is identified with REE3+point defects.
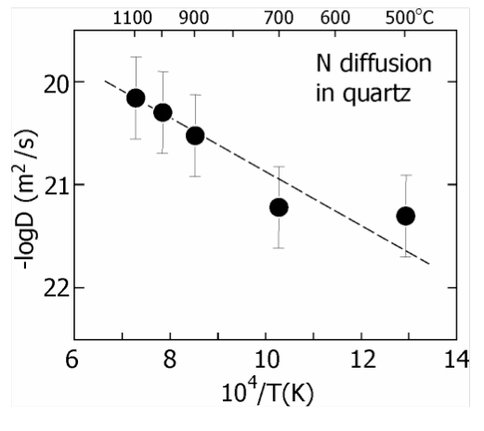
Uptake and diffusion of atmospheric gases in rock-forming minerals. The objective of this subproject is to evaluate the possibility that minerals at or near the surface of the early Earth surface might incorporate gas molecules representative of the terrestrial atmosphere at the time. Our initial experiments have focused upon quartz because this is the dominant matrix material of the ~3.0 to 4.4 Gyr-old Jack Hills zircons. We are examining nitrogen uptake first because of its abundance in today’s atmosphere. This greatly simplifies the initial experiments, which have involved simple heating of natural quartz crystals in air and measuring nitrogen uptake. We have characterized N diffusive- uptake profiles using the nuclear reaction 14N(α,p)17O. Results to date reveal that a significant amount of nitrogen (~1000 ppm) is incorporated into the quartz structure, probably in defect sites. Nitrogen diffusion is quite slow, which is an encouraging result because preservation (retention) of N in quartz over vast expanses of geologic time becomes plausible. Figure 2 shows N diffusivity as a function of temperature in a conventional Arrhenius plot. IN order to evaluate N uptake in natural settings, we have examined two natural quartz specimens from Phanerozoic rocks (Arkansas 'rock crystal’and Herkimer 'diamonds’ from New York State) and demonstrated that these have 'captured’ natural nitrogen on the outer ~200 nm of the crystals. The next step in this line of inquiry is evaluate the range of natural conditions under which N is taken up in quartz.
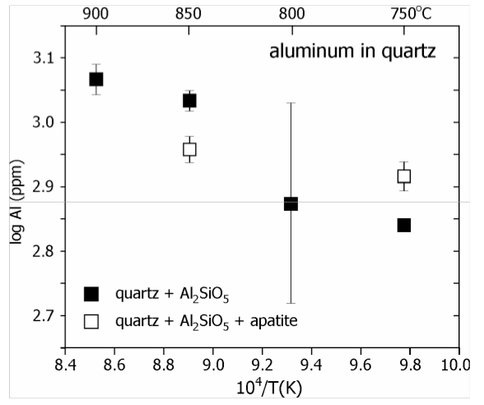
Figure 3. Summary of data on alumi-num incorporation into synthetic quartz crystallized in the presence of Al2SiO5 (a likely phase in metamorphic rocks formed from clay-rich sediment). All experiments were conducted at 1 GPa.
Incorporation of aluminum into quartz. As noted in the previous section of our report devoted to trapping of atmospheric gases in minerals, quartz has been an important mineral in the Earth’s crust as far back as the early Archean, and possibly even the Hadean Eon (prior to 3.8 Gyr). This ubiquitous mineral crystallizes in many environments, including low-temperature hydrothermal veins, granites and other felsic intrusives, rhyolites, pegmatites, and in metamorphic rocks of many grades and compositions. Thus the opportunity exists to shed light on the nature of the earliest continental crust by developing tools to determine the crystallization environment of ancient quartz. We believe that this can be done by calibrating the extent to which various impurities (e.g., Ti, Al, Fe, Ca) enter the quartz lattice at given P-T-x conditions. Recent efforts in E.B. Watson’s group have focused upon calibrating Ti incorporation in quartz, which now can be used to determine the temperature and/or pressure of quartz crystallization. In the new Rensselaer Astrobiology Center we have turned our attention to other impurities in quartz that may provide additional information on the circumstances and chemical environment of quartz crystallization, with the ultimate goal of applying the results to ‘reverse engineer’ the ancient quartz grains of the Jacks Hills and other early Archean sediments. We began with a calibration of aluminum uptake into the quartz structure. Quartz in metapelite (a metamorphic rock of clay-rich precursor sediment) is likely to coexist with an aluminosilicate phase (Al2SiO5), which would fix the chemical activity of Al2O3 at a high value, and our initial experiments were designed to simulate this condition. Figure 3 shows our preliminary results for the aluminum incorporation in quartz coexisting with Al2SiO5: the solubility of Al is substantial (>1000 ppm at 900°C) and easily measured by electron microprobe. We will pursue a systematic study of Al uptake during the months ahead and also explore the potential of Fe impurities in quartz to yield secrets about prevailing oxygen pressure in ancient environments.
Figure 2. Summary of data on diffusion of nitrogen into quartz between 500 and 1100°C (D is the diffusivity of N in m2/s). The data were obtained simply by heating quartz in air and measuring the N diffusive uptake profiles using the 14N(α,p)17O reaction. Nitrogen penetrates ~50-100 nm into the quartz at the conditions of the experiments; the concentration at the quartz
surface is ~1000 ppm. For a diffusivity of 10-22 m2/s (~500°C), the diffusive length scale in a natural setting would be a few hundred microns.
Publications
- Trail, D., Thomas, J.B. & Watson, E.B. (2009). OH in zircon. Geochim. Cosmochim. Acta, 73(13): A1343.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Suzanne Baldwin
Co-Investigator
John Delano
Co-Investigator
Thomas Mueller
Postdoc
Nick Tailby
Postdoc
Dustin Trail
Graduate Student
Sebastian Mergelsberg
Undergraduate Student
Egidio Tentori
Undergraduate Student
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 4.1
Earth's early biosphere.
Objective 4.3
Effects of extraterrestrial events upon the biosphere