2009 Annual Science Report
 Montana State University
Reporting | JUL 2008 – AUG 2009
Montana State University
Reporting | JUL 2008 – AUG 2009
Probing the Structure and Nitrogen Reduction Activity of Iron-Sulfur Minerals
Project Summary
Iron-sulfur compounds are common in both biological and geological systems. The adaptation of Fe-S clusters from the abiotic world to the biological world may have been an early event in the development of life on Earth and possibly a common feature of life elsewhere in the universe. The iron-sulfur mineral thrust of the ABRC is focused on examining the structure and reactivity of FeS minerals using nitrogen fixation as a model reaction.
Project Progress
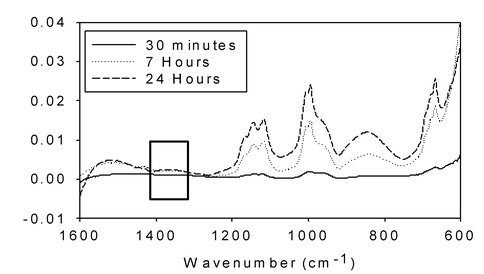
The Strongin group is continuing to study the reduction of dinitrogen to ammonia driven by the conversion of pyrrothite to pyrite (FeS + H2S → FeS2 + 2H+ + 2e-). In addition the group has initiated parallel studies that are investigating the conversion of nitrate and nitrite to ammonia. The experiments are utilizing a transmission infrared cell containing an aqueous suspension of nanocrystalline FeS, sulfide species, and dissolved nitrogen at 120°C and 100 bar N2 pressure. Vibrational spectroscopy has suggested that ammonia is being formed under these conditions and the details of the data are being currently analyzed so as to understand the mechanistic steps that contribute to this reaction chemistry. FeS surfaces modified by exposure to an energetic hydrogen beam by the Minton group will also be studied in the near future. These modified surfaces contain a more reduced form of iron (than in FeS) that may enhance ammonia formation under hydrothermal conditions.
The spectroscopic studies are complemented by batch experiments carried out at Stony Brook in Schoonen’s group. The batch experiments are designed to derive kinetic data and activation energies. Experiments with nitrate and freshly precipitated FeS—precipitated from FeSO4 or FeCl2 salts—do not show any formation of ammonia, consistent with the spectroscopic studies. Work with nitrite, which is more reactive is underway. In addition to the experimental work on reactions with FeS, Post Doctoral researcher Alex Smirnov is collaborating with a post-doctoral researcher at Carnegie to study the occurrence of nitride phases in several iron meteorites. The objective is to determine their concentration and rate of leaching from meteorites so an estimate can be made of exogenous addition of reduced nitrogen to the Hadean Earth.
In situ transmission FTIR spectra of FeS slurry exposed to water equilibrated with N2 at 50 atm. Experiment conducted at 120ºC. Box outlines the spectral region characteristic for ammonia. Changes in the spectra below 1200 wavenumbers are due to formation of NOx SOx, and HSOx
Publications
-
Murphy, R., & Strongin, D. (2009). Surface reactivity of pyrite and related sulfides. Surface Science Reports, 64(1), 1–45. doi:10.1016/j.surfrep.2008.09.002
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Alexander Smirnov
Postdoc
Riley Murphy
Graduate Student
Alex Gordon
Unspecified Role
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems