2009 Annual Science Report
 Montana State University
Reporting | JUL 2008 – AUG 2009
Montana State University
Reporting | JUL 2008 – AUG 2009
Molecular Beam Studies of Nitrogen Reactions on Iron-Sulfur Surfaces
Project Summary
It is generally accepted that surface-mediated reactions occur on defect sites. The role of defects in the formation of ammonia is being systematically evaluated using molecular beam-surface scattering experiments in which a hydrogen atom plasma source (deuterium due to easier detection) is used to hydrogenate a pyrite surface. The hydrogenated surface is subsequently bombarded with a molecular beam of energetic nitrogen molecules and the conversion of nitrogen to products, such as ammonia is probed through mass spectrometry.
Project Progress
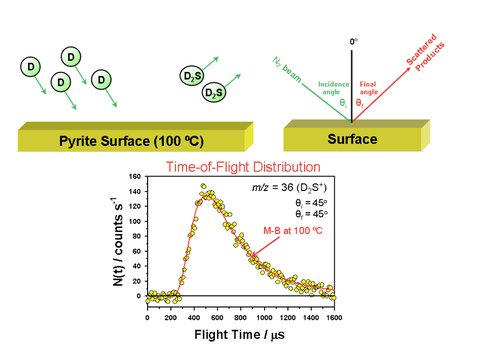
Recent work has focused on the initial mapping of the enormous parameter space that is available in these beam/surface scattering experiments. As the first step, the preparation and characterization of a model iron-sulfur surface was carried out, which is to be used in subsequent experiments on reactivity. We have discovered that hydrogen atoms can be used to reduce a pyrite surface to produce a reduced iron-sulfur surface which contains formally Fe(I) centers with similarities to the co-factor in hydrogenase and nitrogenase. The reduced iron site is likely to be an active surface for ammonia synthesis. Work that led to this discovery involved strong cooperation between Minton and Szilagyi. In the Minton lab, D atoms, produced by an RF plasma and expanded in an effusive beam, were directed at a pyrite [100] surface held at various temperatures and for different exposure times. During exposure, D2S products were detected by a mass spectrometer and had a thermal distribution of molecular speeds, indicating that the D atoms became trapped on the surface before reacting with S atoms. The yield of D2S from the surface decreased with exposure time, indicating an increasingly iron-rich surface. As the surface was modified by the D-atom beam, a second beam of N2 molecules was directed at the surface, and the behavior of the inelastically scattered N2 indicated that the surface became rougher during the exposure; this conclusion was verified by surface characterization using scanning electron microscopy. The detailed understanding of the modified surface came from Szilagyi’s multi-edge X-ray absorption studies, which demonstrated clearly that the higher temperature surfaces (~ 200 ºC) were reduced from formally Fe(II) to Fe(I). Below hydrothermal temperatures (less than 100 ºC), they were not able to detect any considerable amount of the reduced iron-sulfur phase. Thus, the rate of surface transformation appears to be strongly temperature dependent. The reduced surface is stabilized in air by the formation of a passivating oxide layer that is identical to the layer covering metallic iron powder. Our immediate goals include the preparation of such reduced surfaces in vacuum and investigation of ammonia formation by directing beams of N2 and H atoms at the surfaces and detecting the product NH3 by mass spectrometry.
Reactive Scattering of D2S on a Pyrite Surface: D2S exits the surface with a Maxwell-Boltzmann distribution of velocities characteristic of the surface temperature indicating that reaction occurs in thermal equilibrium with the surface
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Martin Schoonen
Co-Investigator
Daniel Strongin
Co-Investigator
Robert Szilagyi
Co-Investigator
Alexios Grigoropoulos
Postdoc
Che Li
Postdoc
Alexander Smirnov
Postdoc
Riley Murphy
Graduate Student
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems