2009 Annual Science Report
 Montana State University
Reporting | JUL 2008 – AUG 2009
Montana State University
Reporting | JUL 2008 – AUG 2009
Evolution of Nitrogen Fixation, Photosynthesis, Hydrogen Metabolism, and Methanogenesis
Project Summary
We have developed a new line of investigation to complement our work on the biochemistry of complex iron-sulfur cluster enzyme structure, function and biosynthesis with the aim of probing complex iron-sulfur enzyme evolution. We are studying the phylogenetic trajectory of multiple genes involved in complex iron-sulfur cluster function and biosynthesis to probe the evolutionary origin of aspect of hydrogen metabolism and modes of biological nitrogen fixation.
Project Progress
As a complement to our work on complex iron-sulfur cluster structure, function, and biosynthesis, we developed a complementary line of inquiry probing evolutionary relationships of complex iron-sulfur enzymes. We are using a new approach to phylogenic analysis that investigates in tandem the phylogenetic trajectories of suites of genes involved in aspects of hydrogen metabolism, nitrogen fixation, and photosynthesis to evaluate their evolutionary relationships and evolutionary origins. We are exploiting the complexity of these processes and the requirement for the concerted action of multiple gene products in addition to the structural genes to confer function. In a number of cases the evolutionary relationships of gene products involved have arisen from defined genetic events, such as gene duplications and/or gene fusions that can be placed definitively in the evolutionary record.
The results of these studies have already produced some exciting insights. In one study we investigated the evolutionary origin of biological nitrogen fixation and have deduced that biological nitrogen fixation as we know it was not associated with the last universal common ancestor. Additionally the analysis indicates that the alternative (iron-only and vanadium) forms emerged prior to molybdenum-containing nitrogenase and the molybdenum nitrogenase originated in the methanogenic archaea. Further molecular clock analysis allows us to place the origin of Mo-nitrogenase in geologic time relative to the advent of oxygenic photosynthesis and the ‘Great Oxidation Event’.
Applying this approach to hydrogen evolution has resulted in defining evolutionary relationships between microbial [FeFe]-hydrogenases and the related Nar1p family of proteins involved in eukaryotic iron-sulfur cluster biosynthesis. We have made significant progress in examining the evolutionary relationships between various forms of [NiFe]-hydrogenases and complex I of the respiratory chain. In addition, we have been able to implicate new genes involved in cluster biosynthesis through their shared patterns of inheritance with known gene products.
A). Co-localization of hmdABC in the genomes of hydrogenotrophic methanogens. B). Phylogenetic reconstruction of HmdA and HmdB indicate similar topologies, indicating that the protein-encoding genes co-evolved. C). Phylogenetic reconstruction of representatives of the radical SAM superfamily of enzymes indicates that HmdB is related to BioB and HydE.
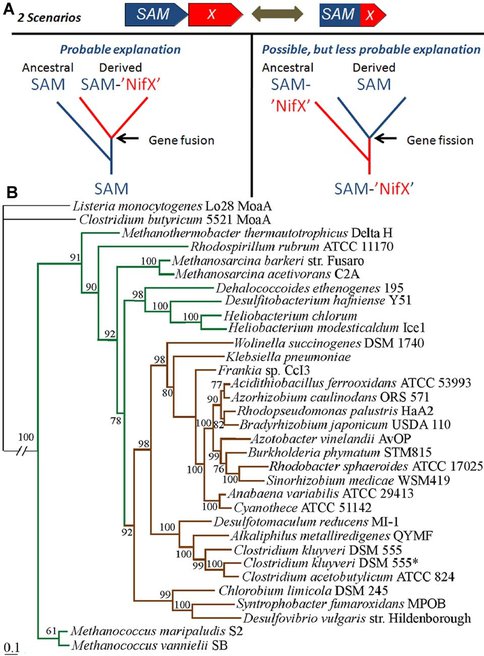
It is not known whether the ancestral state of NifB contained the fused the radical SAM domain (SAM) and the NifX domain (X) or just the SAM domain; however, it is most probable that the ancestral state contained only the SAM domain. B). Phylogenetic reconstruction of just the SAM domain indicates that the early emerging lineages contain only the SAM domain (green) and recently evolved lineages have fused SAM-NifX proteins (brown).
Publications
-
McGlynn, S. E., Boyd, E. S., Shepard, E. M., Lange, R. K., Gerlach, R., Broderick, J. B., & Peters, J. W. (2009). Identification and Characterization of a Novel Member of the Radical AdoMet Enzyme Superfamily and Implications for the Biosynthesis of the Hmd Hydrogenase Active Site Cofactor. Journal of Bacteriology, 192(2), 595–598. doi:10.1128/jb.01125-09
-
Mulder, D. W., Boyd, E. S., Sarma, R., Lange, R. K., Endrizzi, J. A., Broderick, J. B., & Peters, J. W. (2010). Stepwise [FeFe]-hydrogenase H-cluster assembly revealed in the structure of HydAΔEFG. Nature, 465(7295), 248–251. doi:10.1038/nature08993
- Boyd, E.S., Anbar, A.D., Miller, S., Hamilton, T.L., Lavin, M. & Peters, J.W. (Submitted). An early origin for Molybdenum-nitrogenase. Science.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Joan Broderick
Co-Investigator
Eric Boyd
Postdoc
Trevor Beard
Graduate Student
Trinity Hamilton
Graduate Student
-
RELATED OBJECTIVES:
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems