2009 Annual Science Report
 Georgia Institute of Technology
Reporting | JUL 2008 – AUG 2009
Georgia Institute of Technology
Reporting | JUL 2008 – AUG 2009
High Level Theory - the Role of Mg2+ in Ribosome Assembly
Project Summary
We have embarked on a computational evaluation of the role of Mg2+ microclusters observed to form a scaffold for the extant and ancestral peptidyl transferase center. The interaction energies of ribosomal RNA with single and multiple Mg2+ cations are computed, and deconvolved. The results will be compared to those with other metals, to determine why Mg2+ plays a special role in RNA folding.
Project Progress
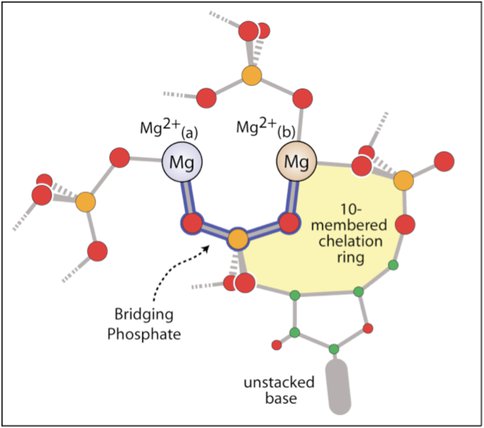
It has been shown by Hsiao and coworkers1,2 that three Mg2+-microsclusters (Mg2+-μc’s), each containing two Mg2+ ions, provide a rigid framework surrounding the peptidyl transferase center of the ribosome. A schematic representation of a generic Mg2+-μc is shown in Figure 1. A Mg2+-μc extracted from ribosomal structures is shown in Figure 2. We have initiated a high level theoretical evaluation of the contribution of Mg2+-μc’s to the energetics of ribosomal RNA assembly, using NASA supercomputing facilities. We will deconvolve the energy of interaction into fundamental components. Initial calculations using Gaussian30 were performed on ten-membered ring complexes (shaded yellow in Figures 1 and 2)
Figure 1. Figure 1. A schematic diagram of common features of Mg2+-mc architecture. Shown is the Mg2+(a)-(O1P-P-O2P)-Mg2+(b) bridge (outlined in blue), the 10-membered ring (yellow), an unstacked base, and the additional RNA ligands that enter the Mg2+ first shell at variable positions. Carbon is green, oxygen is red, and phosphorous is orange.
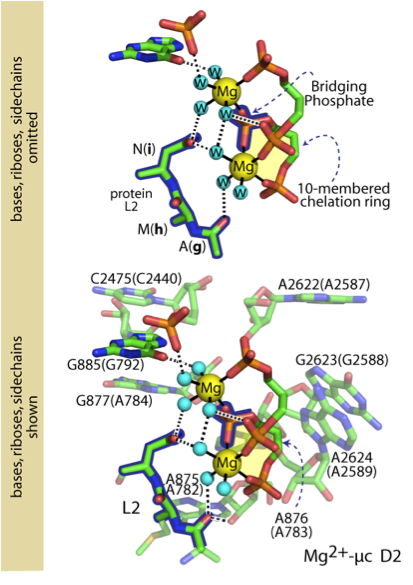
Figure 2. Figure 2. A Mg2+-mc shown at two levels of detail. Both H. marismortui and (E. coli) residue numbers are shown.
consisting of a nucleic acid fragment with a sugar (ribose or deoxyribose), two phosphate groups, a Mg2+ ion coordinated by two phosphate oxygens, and four water molecules (waters not shown in Figure 1). Geometry was optimized in the gas phase using Density Functional Theory. We incorporated solvent effects using the polarized continuum models (PCMs) at the BLYP/6-311++(d,p) level of theory. Ultimately we will characterize intact Mg2+-μc’s consisting of 120 atoms, including hydrogen atoms and multiple ions.
We have computed the interaction energy of the ten-membered ring complex, for both ribose and deoxyribose analogs, and found that formation of such complexes is energetically favorable (-5.6 kcal/mol for ribose and -3.4 kcal/mol for deoxyribose). We found a very high energetic penalty associated with the closing the sugar-phosphate backbone (~ 45 kcal/mol) to form the ring. We are repeating the simulations for complexes in which the same nucleic acid fragments are bound to Na/ins>, Ca2+ and Fe2+ in place Mg2+. Our hypothesis is that Mg2+ is can be replaced by Fe2+ but not Na+ or Ca2+ in forming a stable ribosomal core.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Anton Petrov
Postdoc
-
RELATED OBJECTIVES:
Objective 3.2
Origins and evolution of functional biomolecules
Objective 5.3
Biochemical adaptation to extreme environments