2015 Annual Science Report
 VPL at University of Washington
Reporting | JAN 2015 – DEC 2015
VPL at University of Washington
Reporting | JAN 2015 – DEC 2015
Jon Toner NAI NPP Postdoc Report
Project Summary
Aqueous salt solutions are critical for understanding the potential for liquid water to form on icy worlds and the presence of liquid water in the past. Salty solutions can form potentially habitable environments by depressing the freezing point of water down to temperatures typical of Mars’ surface or the interiors of Europa or Enceladus. We are investigating such low-temperature aqueous environments by experimentally measuring the low temperature properties of salt solutions and developing thermodynamic models to predict salt precipitation sequences during either freezing or evaporation. These models, and the experimental data we are generating, are being applied to understand the conditions under which water can form, the properties of that water, and what crystalline salts indicate about environmental conditions such as pH, temperature, pressure, and salinity.
Project Progress
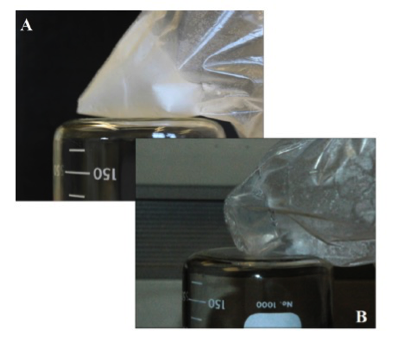
Supercooling in Salt Solutions: Toner and colleagues studied how much salt solutions will supercool before crystallizing either salt or ice (Toner et al., 2014a). They found that salt solutions supercool to a remarkable degree, typically ~15°C below equilibrium predictions. Perchlorate-rich (ClO4) salt solutions tend to supercool much more than analogous chlorides and sulfates. In particular, Mg(ClO4)2 and Ca(ClO4)2 solutions will supercool down to -120°C, and then transition into an amorphous glass (Figure 1). Such glasses are potentially important for astrobiology because glasses preserve organic molecules and structures, such that organisms preserved in glasses can remain viable upon rewarming.
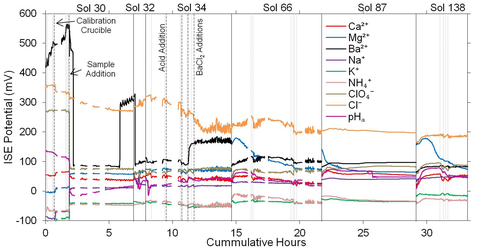
Reanalysis of Phoenix Lander Data: Toner and colleagues also reanalyzed data from the Wet Chemistry Laboratory (WCL) on the Mars Phoenix Lander in order to better constrain absolute concentrations of salts in Martian soils, and uncertainties in the WCL data (Toner et al., 2014b). Improvements to original analyses included Kalman optimal smoothing (for smoothing noise in the data) and corrections for the formation of ion-pairs in solutions, such as MgSO4 (Fig. 2). We then took our revised analysis and modeled equilibrium freezing and evaporation of a nominal WCL solution using the geochemical model FREZCHEM and a chemical-divide model. Our model results indicate that Mg-Na-ClO4-rich solutions evolve during freezing and that a variety of primarily Mg-Na-rich sulfates, chlorides, and perchlorates form at the solution eutectic near 200 K.
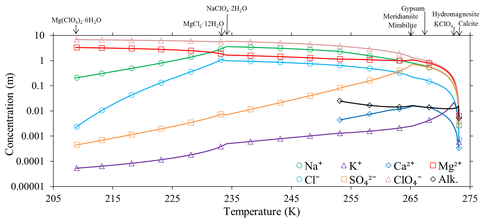
Modeling Freezing and Evaporation of Perchlorate Salts on Mars: The modeling work by Toner and others on the WCL data reanalysis shows that FRZCHEM, a model commonly used to study freezing/evaporation of salt solutions, often makes predictions that are clearly in error when modeling complex salt mixtures. To develop a working multicomponent aqueous model, we parameterized a Pitzer model at 298 K using compilations of solubility data in aqueous salt mixtures (Toner et al., 2015a). The resulting model is a significant improvement over FREZCHEM. We then extended this model to <200 K using experimental solubilities in perchlorate solutions that we measured in our laboratory using a custom built cryogenic vessel (Toner et al., 2015b). To determine the full range of salt precipitates that might occur on Mars, we modeled evaporation of WCL solutions from 298 K to the eutectic at 209 K (Fig. 3). Our model results indicate that potentially habitable brines, defined as having a water activity greater than 0.6, can occur only above 219 K and 0.4 wt. % water. Overall, our modeling results in this study outline a roadmap of soluble mineral assemblages that are possible under different temperature and soil water content regimes, and can be used to interpret both past and future identifications of soluble minerals on Mars.
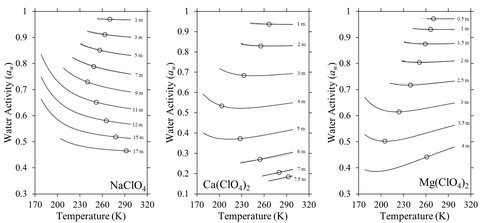
Water activities in perchlorate brines: Perchlorate salts found on Mars are extremely hygroscopic and form low eutectic temperature aqueous solutions, which could allow liquid water to exist on Mars despite cold and dry conditions. The formation, dynamics, and potential habitability of perchlorate salt solutions can be broadly understood in terms of water activity. Water activity controls condensation and evaporation of water vapor in brines, deliquescence and efflorescence of crystalline salts, and ice formation during freezing. Furthermore, water activity is a basic parameter defining the habitability of aqueous solutions. Despite the importance of water activity, its value in perchlorate solutions has only been measured at 298.15 K and at the freezing point of water. To address this lack of data, we have determined water activities in NaClO4, Ca(ClO4)2, and Mg(ClO4)2 solutions using experimental heat capacities measured by Differential Scanning Calorimetry (Toner and Catling, 2016). We find that water activities in NaClO4 solutions increase with decreasing temperature, by as much as 0.25 aw from 298.15 to 178 K (Fig. 4). Consequently, aw reaches ~0.6-0.7 even for concentrations up to 15 molal NaClO4 below 200 K. In contrast, water activities in Ca(ClO4)2 and Mg(ClO4)2 solutions generally decrease with decreasing temperature. The temperature dependence of water activity indicates that low-temperature NaClO4 solutions will evaporate and deliquesce at higher relative humidity, crystallize ice at higher temperature, and potentially be more habitable for life (at least in terms of water activity) compared to solutions at 298.15 K. The opposite effects occur in Ca(ClO4)2 and Mg(ClO4)2 solutions.
Publications
-
Toner, J. D., Catling, D. C., & Light, B. (2015). A revised Pitzer model for low-temperature soluble salt assemblages at the Phoenix site, Mars. Geochimica et Cosmochimica Acta, 166, 327–343. doi:10.1016/j.gca.2015.06.011
-
Toner, J. D., Catling, D. C., & Light, B. (2015). Modeling salt precipitation from brines on Mars: Evaporation versus freezing origin for soil salts. Icarus, 250, 451–461. doi:10.1016/j.icarus.2014.12.013
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
David Catling
Co-Investigator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments