2015 Annual Science Report
 University of Wisconsin
Reporting | JAN 2015 – DEC 2015
University of Wisconsin
Reporting | JAN 2015 – DEC 2015
Project 3C: A Redox-Stratified Ocean 3.2 Billion Years Ago
Project Summary
A novel combination of stable Fe and radiogenic U–Th–Pb isotope data that demonstrate that significant oxygen contents existed in the shallow oceans at 3.2 Ga, based on analysis of the Manzimnyama Banded Iron Formation (BIF), Fig Tree Group, South Africa. This unit is exceptional in that proximal, shallow-water and distal, deep-water facies are preserved. When compared to the distal, deep-water facies, the proximal samples show elevated U concentrations and moderately positive 56Fe values, indicating vertical stratification in dissolved oxygen contents. Confirmation of oxidizing conditions using U abundances is robustly constrained using samples that have been closed to U and Pb mobility using U–Th–Pb geochronology. This documents the oldest known preserved marine redox gradient in the rock record. The relative enrichment of O2 in the upper water column is likely due to the existence of oxygen-producing microorganisms such as cyanobacteria. These results provide a new approach for identifying free oxygen in Earth’s ancient oceans, including confirming the age of redox proxies, and indicate that cyanobacteria evolved prior to 3.2 Ga.
Project Progress
The timing of oxygenation of the world’s oceans and atmosphere has long been a topic of debate. A long-standing proposal embraced by many studies is that a step-wise oxygenation of the planet occurred, with the first rise in oxygen taking place between 2.4 and 2.2 billion years ago (Ga), a time referred to as the Great Oxidation Event (GOE), and a second rise approximately 0.6 billion years ago to nearly present-day levels (Holland, 1984). The world before the GOE is thought to have been largely anoxic, where oxygen sinks out-paced oxygen sources. Recent studies, however, have called into question this strictly step-wise increase in planetary oxygen levels, instead proposing transient increases in atmospheric oxygen before the GOE as far back as 3.0 Ga (e.g., Crowe et al., 2013). In contrast, using combined U–Pb and Fe isotope data on the 3.4 Ga Marble Bar Chert jasper (Pilbara Craton, Australia), we previously argued that the oceans at that time were essentially anoxic (Li et al., 2013). Although there is an emerging view that intermittent rises in oxygen likely occurred prior to the GOE (Lyons et al., 2014), little is known about the potential transitions in oxygen contents in seawater that may have occurred over the 400 Myr between deposition the Marble Bar Chert and sedimentary rocks in the Pongola Basin.
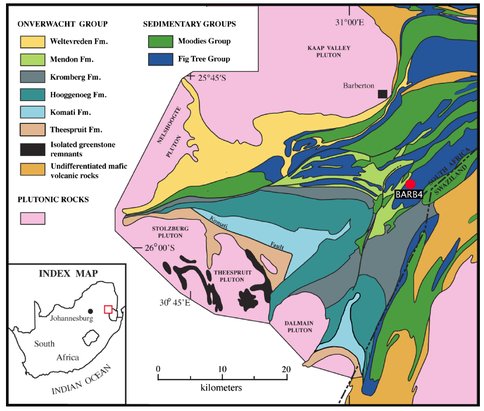
In this project, we combined U–Th–Pb geochronology and stable Fe isotope results from the 3.23 Ga Manzimnyama BIF of the Fig Tree Group (Barberton greenstone belt, South Africa). Samples are from the BARB4 diamond drilled scientific core (Figure 1), and include high-Fe and low-Fe cherts that record distinct depositional conditions which allow evaluation of redox conditions from deep- and shallow-ocean environments, respectively. Deep Archean seawater is generally thought to have been anoxic (Kamber et al., 2014), however, if oxygen was being produced by oxygenic photosynthesis, this production would likely happen in shallow water (photic zone) where cyanobacteria would thrive. Thus, the preservation of distinct deep- and shallow-water facies allows us to compare anoxic deep waters with potentially more oxygen enriched shallow waters.
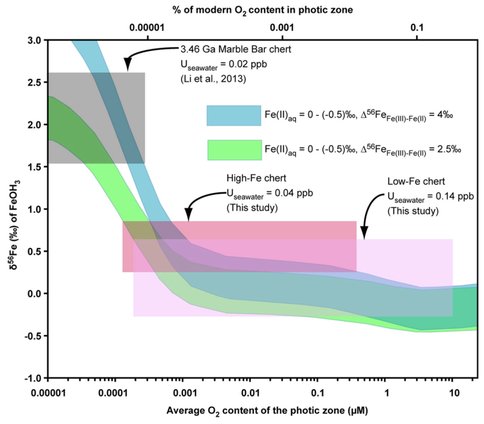
Using a reaction-transport model (Li et al., 2013), an estimate may be made for the oxygen contents in the shallow ocean based on the Fe isotope compositions for iron oxides. It is not possible to quantify oxygen contents based on U abundances because U contents are not limited by mineral solubility in modern seawater, and this seems likely to have been the case in the Archean. Use of Fe isotope compositions for iron oxides to constrain ocean water oxygen contents suggests that the high-Fe chert samples could have formed from seawater that had a maximum oxygen content of 0.4 micromolar, whereas the low-Fe chert samples could have formed from seawater that had a maximum oxygen content of ~10 micromolar (Figure 2). The ~10 micromolar oxygen content is the maximum predicted during the Archean based on modeling possible oxygen levels in the shallow oceans beneath an anoxic (Olson et al., 2013). Although it is difficult to confidently constrain oxygen contents using Fe isotope composition for iron oxides as the isotopic composition approaches that of the hydrothermal source, the difference in average Fe isotope compositions from the shallow-water (low-Fe) and deep-water (high-Fe) cherts is consistent with the relative redox state inferred from the U abundances. Moreover, the modeling in Figure 2 indicates that oxygen contents at 3.23 Ga were significantly higher than that inferred for 3.46 Ga based on the Marble Bar Chert (Figure 2).
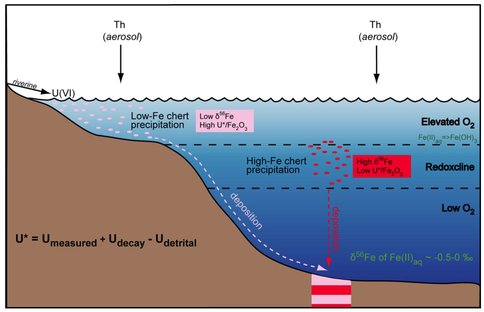
In Figure 3 we illustrate a conceptual model for redox zonation in the oceans as recorded by the 3.23 Ga Manzimnyama BIF. Although oxidation of hydrothermally sourced Fe(II) can be accomplished through anaerobic photoautotrophic microorganisms, which use aqueous Fe(II) as an electron (Widdel et al., 1993), or by free oxygen, the elevated U abundances we calculate for the shallow oceans argues the oxidation mechanism was through free oxygen. Some oxidative weathering on the continents must have occurred to transport soluble U(VI) to the oceans, although such weathering may have been quite low, and may have limited U(VI) availability. The co-variation in U in seawater and Fe isotope compositions between proximal (low-Fe) and distal (high-Fe) samples indicates that oxygen was enriched in the photic zone. We propose that the proximal (low-Fe) samples precipitated largely above the redoxcline in shallow water, whereas the distal (high-Fe) samples precipitated from a larger portion of the water column that included the upper, more oxygen-rich zone, but also lower parts of the water column where oxygen contents would be lower (Figure 3). Based on comparison with results from the 3.46 Ga Marble Bar Chert (MBC), Australia, a redoxcline formed in seawater after 3.46 Ga but before 3.23 Ga. Oxygenic photosynthesis by cyanobacteria has been suggested as a mechanism for producing oxygen at 2.8 Ga (Riding et al., 2014) and 3.0 Ga for the Pongola Basin (Crowe et al., 2013; Planavsky et al., 2014), and we suggest the same as a mechanism for oxygen generation here. The work presented here, therefore, pushes back the earliest evidence for oxygenic photosynthesis by ~200 million years.
References Cited
Crowe, S.A., Døssing, L.N., Beukes, N.J., Bau, M., Kruger, S.T., Frei, R., Canfield, D.E., 2013. Atmospheric oxygenation three billion years ago. Nature 501, 535–539.
Holland, H.D., 1984. The Chemical Evolution of the Atmosphere and Oceans. Princeton University Press.
Kamber, B.S., Webb, G.E., Gallagher, M., 2014. The rare earth element signal in Archean microbial carbonate: information on ocean redox and biogenicity. J. Geol. Soc. 171, 745–763.
Li, W., Czaja, A.D., Van Kranendonk, M.J., Beard, B.L., Roden, E.E., Johnson, C.M., 2013. An anoxic, Fe(II)-rich, U-poor ocean 3.46 billion year ago. Geochim. Cosmochim. Acta 120, 65–79.
Lyons, T.W., Reinhard, C.T., Planavsky, N.J., 2014. The rise of oxygen in the Earth’s early atmosphere. Nature 506, 307–315.
Olson, S.L., Kump, L.R., Kasting, J.F., 2013. Quantifying the areal extent and dissolved oxygen concentrations of Archean oxygen oases. Chem. Geol. 362, 35–43.
Planavsky, N.J., Asael, D., Hofmann, A., Reinhard, C.T., Lalonde, S.V., Knudsen, A., Wang, X., Ossa Ossa, F., Recoits, E., Smith, A.J.B., Beukes, N.J., Bekker, A., Johnson, T.M., Konhauser, K.O., Lyons, T.W., Rouxel, O.J., 2014. Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nat. Geosci. 7, 283–286.
Riding, R., Fralick, P., Liang, L., 2014. Identification of an Archean marine oxygen oasis. Precambrian Res. 251, 232–237.
Widdel, F., Schnell, S., Heising, S., Ehrenreich, A., Assmus, B., Schink, B., 1993. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature 362, 834–836.
Publications
-
Satkoski, A. M., Beukes, N. J., Li, W., Beard, B. L., & Johnson, C. M. (2015). A redox-stratified ocean 3.2 billion years ago. Earth and Planetary Science Letters, 430, 43–53. doi:10.1016/j.epsl.2015.08.007
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Co-Investigator
Aaron Satkoski
Collaborator
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 5.2
Co-evolution of microbial communities
Objective 7.1
Biosignatures to be sought in Solar System materials