2015 Annual Science Report
 University of Wisconsin
Reporting | JAN 2015 – DEC 2015
University of Wisconsin
Reporting | JAN 2015 – DEC 2015
Project 2C: Investigation of the Role of Polysaccharide in the Dolomite Growth at Low Temperature by Using Atomistic Simulations
Project Summary
Polysaccharides in microbial EPS can promote dolomite growth at room temperature. Our molecular dynamics modeling results show that adsorbed polysaccharides can lower activation energy for removing surface water molecules next to the polysaccharides and catalyze dolomite crystallization at low temperature. The process can lower the energy barrier by ~ 1 kcal / mole. Low temperature dolomite / sedimentary dolomite is a potential biosignature. The new finding also provides key to solving the “Dolomite Problem” that has puzzled geologists for decades.
Project Progress
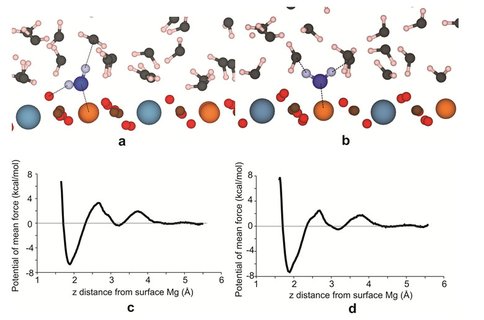
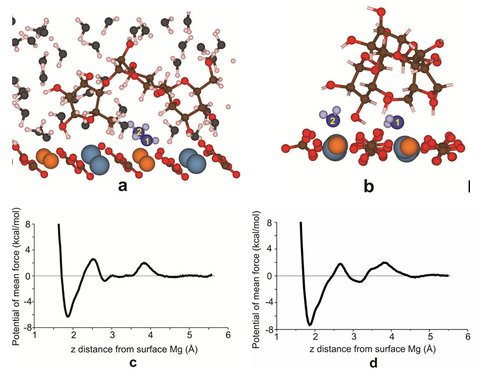
Dehydration of water from surface Mg2+ is most likely the rate-limiting step in the dolomite growth at low temperature. Here, we investigate the role of polysaccharide in this step using classical molecular dynamics (MD) calculations. Free energy (potential of mean force, PMF) calculations have been performed for water molecules leaving the first two hydration layers above the dolomite (104) surface under the following three conditions: without catalyst (Fig. 1), with monosaccharide (mannose) and with oligosaccharide (three units of mannose) (Fig. 2). MD simulations reveal that there is no obvious effect of monosaccharide in lowering the dehydration barrier for surface Mg2+. However, we found that there are metastable configurations of oligosaccharide, which can decrease the dehydration barrier of surface Mg2+ by about 0.7-1.1 kcal/mol. In these configurations, the molecule lies relatively flat on the surface and forms a bridge shape. The hydrophobic space near the surface created by the non-polar –CH groups of the oligosaccharide in the bridge conformation is the reason for the observed reduction of dehydration barrier.
Our simulations have focused on the dehydration of surface Mg and we have shown that the polysaccharide is able to decrease the dehydration barrier for the surface Mg by its hydrophobic –CH groups, which is a possible reason why polysaccharide is important in the dolomite growth at low temperature. Nevertheless, the pathway for carbonate ions to supplant the surface water molecules in the presence of polysaccharide is complex and needs to be further investigated.
Publications
-
Shen, Z., Szlufarska, I., Brown, P. E., & Xu, H. (2015). Investigation of the Role of Polysaccharide in the Dolomite Growth at Low Temperature by Using Atomistic Simulations. Langmuir, 31(38), 10435–10442. doi:10.1021/acs.langmuir.5b02025
-
Zhang, F., Xu, H., Shelobolina, E. S., Konishi, H., Converse, B., Shen, Z., & Roden, E. E. (2015). The catalytic effect of bound extracellular polymeric substances excreted by anaerobic microorganisms on Ca-Mg carbonate precipitation: Implications for the “dolomite problem”. American Mineralogist, 100(2-3), 483–494. doi:10.2138/am-2015-4999
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Philip Brown
Collaborator
Izabela Izabela Szlufarska
Collaborator
Zhizhang Shen
Collaborator
-
RELATED OBJECTIVES:
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems