2015 Annual Science Report
 University of Wisconsin
Reporting | JAN 2015 – DEC 2015
University of Wisconsin
Reporting | JAN 2015 – DEC 2015
Project 2B: Cation Content of Carbonate Minerals as a pCO2 Proxy: Inorganic Synthesis Experiments
Project Summary
Atmospheric CO2 content and temperature control reaction mechanisms and precipitation kinetics for carbonate minerals. As such, these parameters may indirectly influence the geochemistry and isotope composition of carbonate minerals. To better understand the significance of these geochemical fingerprints, a suite of laboratory experiments is being conducted to characterize the effect of PCO2 (<1 to >90% CO2) and aqueous ratios and low mol% MgCO3 (<3). With the completion of upcoming Mg-isotope analyses and the establishment of a unified consensus on Mg isotope fractionation relations in calcite, Mg isotope systematics can be used to better understand the geochemical and environmental information archived in the rock record.
Project Progress
A better understanding of the fundamental factors that control the geochemistry of carbonate minerals is required for geochemical codes to be deciphered accurately from ancient terrestrial and extraterrestrial carbonate deposits. Previous work has documented the dependence of calcite precipitation rate on PCO2 (Busenberg and Plummer 1986). Given that the incorporation of some trace elements in calcite is rate dependent (e.g., Lorens et al., 1981; Dromgoole and Walter, 1990), a PCO2 effect may be expressed as a change in the concentration of a particular trace element (e.g., Fe) in calcite as a consequence of rate effect.
This investigation describes a series of laboratory experiments where fluid chemistry ([Mg] aq/[Ca]aq, [Fe] aq/[Ca]aq and [Mg] aq/[Fe]aq), PCO2 (<1% to 100%), temperature (4° to 80°C), and precipitation rate (101 to 104 micromol m-2 hr-1) are independently controlled during the synthesis of carbonate minerals to establish a PCO2 proxy based on the distribution of cations in the CaCO3-FeCO3-MgCO3 system.
As a collaborative outgrowth of this work, the Mg-isotope composition of the fluids and solids from some experiments will be analyzed to determine the factors that control Mg isotope fractionation during the precipitation of Mg- and Mg-Fe-calcite.
Workplan
Laboratory experiments were designed to test four variables that may affect the cation content of rhombohedral carbonate minerals: 1) temperature, 2) cation:carbonate anion ratio of the fluid, 3) PCO2 of the fluid and 4) [Mg] aq/[Ca]aq, [Fe] aq/[Ca]aq and [Mg] aq/[Fe]aq ratio of the fluid. Experiments are subdivided into three groupings: 1) those that contain Ca as the sole divalent cation that participates in the precipitation reaction. The effect of [Ca]aq/[CO3]aq and PCO2 on the precipitation kinetics of calcite will be evaluated in these experiments; 2) those that contain Mg as an additional divalent cation in solution. In addition to the effect of cation/CO3 aq and PCO2, the effect of [Mg] aq/[Ca]aq on the precipitation kinetics and cation content of the solid phase will be investigated; and 3) those that contain Fe2+ as a third aqueous cation that participates in the precipitation of rhombohedral carbonates. These experiments require all reagents and solutions to be contained within an anaerobic chamber to ensure that iron is incorporated as Fe2+ during the precipitation reaction.
Mg-isotope fractionation in the Mg-calcite system
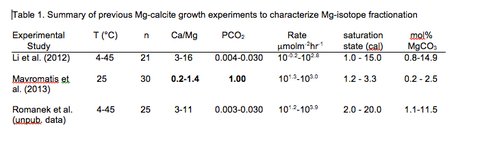
Year one of the project focused on experiments testing the effect of PCO2 and [Mg] aq/[Ca]aq on the precipitation kinetics of Mg-calcite, and in particular the fractionation of Mg-isotopes in the Mg-calcite system. In CAN3, Li et al. (2012) synthesized Mg-calcite (~1-15 mol% MgCO3) in free drift (n=20) and chemostat (n=1) experiments (Table 1).
They determined that Mg isotope fractionation (-2.48 ± 0.15‰) was independent of precipitation rate (10-0.15-102.55 micromol m-2 hr-1) and slightly dependent on temperature (5°- 45°C). Alternatively, Mavromatis et al. (2013) synthesized Mg-calcite at 25°C using the chemostat technique (n=30) and showed that Mg-isotope fractionation displayed a pronounced dependence on precipitation rate (101.26-102.96 micromol m-2 hr-1).
To resolve this discrepancy, Romanek et al. (unpub. data) conducted sixteen additional chemostat experiments between 10° and 45°C, at a PCO2 of 0.3 and 3% and a [Mg]aq/[Ca]aq ratio of 3.3 and 10.0, to determine if a fundamental difference in the free drift and chemostat experiments of Li precluded the expression of a rate effect. Mg-isotope fractionation (-2.53 ± 0.11‰) in these new chemostat experiments was statistically identical to Li’s earlier experiments so some other factor(s) must be responsible for the rate effect reported by Mavromatis.
Two factors that differ substantially between the experiments of Mavromatis and Li/Romanek are PCO2 and [Mg]aq/[Ca]aq. In Mavromatis’ experiments, solution PCO2 was held at 1 atm and [Mg]aq/[Ca]aq was < 0.66 while in the experiments of Li and Romanek solution PCO2 ranged between ~0.04 and 3.0% and [Mg]aq/[Ca]aq ranged between 3.0 and 13.0. Previous studies have documented that solution PCO2 (Busenberg and Plummer, 1986) and [Mg]aq/[Ca]aq (Mucci and Morse 1983) can strongly influence the precipitation kinetics for calcite and it is unclear how these variables may influence Mg-isotope fractionation in the Mg-calcite-[Mg]aq system.
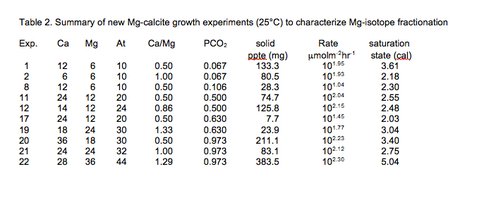
To test whether either of these two variables influence Mg-isotope systematics, twenty additional chemostat experiments were conducted at a solution PCO2 between 0.067 and 0.973 and [Mg]aq/[Ca]aq between 0.5 and 1.3 (Table 2).
Solid precipitates from these experiments are presently being characterized for mol% MgCO3 and Mg isotope composition to determine if rate effect is expressed under these conditions.
Potential Mg hydration effect
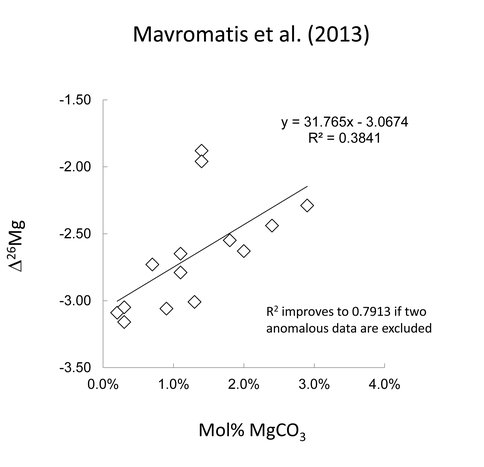
The precipitates from Mavromatis et al. (2013) consisted of calcite having up to 2.5% mol% MgCO3 while the solids of Li and Romanek contained up to 14.9 mol% MgCO3. The relatively low mol% MgCO3 in Mavromatis’ experiments precluded the use of XRD to characterize Mg substitution in the calcite lattice (Zhang et al., 2010) and they characterized the mol% MgCO3 their solids by FTIR and wet chemical analysis. While the two methods were internally consistent, the FTIR analysis showed that Mg was incorporated in the solid as a hydrated phase. Morevover, Mavromatis’ data shows a strong inverse correlation between Mg-isotope fractionation and mol% MgCO3 in the solid (Fig. 1).
This observation presents a third possibility for the expression of a rate effect, i.e., that Mg-isotope fractionation is related to Mg hydration in the calcite lattice. To test for this effect, the solids from Romanek et al. (unpub. data) were analyzed by FTIR to determine the bonding environment for Mg in their precipitates. The FTIR results did not show a relationship between mol% MgCO3 and Mg-hydration, but additional analyses are required at relatively low mol%MgCO3 before definitive conclusions can be drawn.
Workplan for year two
Experiments in year two of the project will focus on the effects of temperature, PCO2 and [Mg]aq/[Ca]aq on the precipitation kinetics of Mg-calcite, and the addition of ferrous iron as an aqueous constituent that participates in formation of rhombohedral carbonates in the CaCO3-FeCO3-MgCO3 system.
References
Busenberg E. and Plummer L.N. 1986 A comparative study of the dissolution and crystal growth kinetics of calcite and aragonite. In Studies in Diagenesis (ed. F.A.Mumpton), U.S. Geological Survey Bull. 1578, 139-168.
Dromgoole E.L and Walter L.M. 1990 Iron and manganese incorporation into calcite-effects of growth-kinetics, temperature and solution chemistry. Chem. Geol. 81, 311-336.
Li W., Chakraborty S., Beard B., Romanek C.S. and Johnson C.M. 2012 Magnesium isotope fractionation during precipitation of inorganic calcite under laboratory conditions. Earth Planet. Science Lett. 333-334, 304-316.
Lorens R.B. 1981 Sr, Cd, Mn and Co distribution coefficients in calcite as a function of calcite precipitation rate. Geochim. Cosmochim. Acta 45, 553-561.
Mavromatis V. Gautier Q., Bosc O. and Schott J. 2013 Kinetics of Mg partition and Mg stable isotope fractionation during its incorporation in calcite. Geochim. Cosmochim.Acta 114, 188-203.
Mucci A.and Morse J.W. 1983 The incorporation of Mg2+ and Sr2+ into calcite overgrowths; influence of growth rate and solution composition. Geochim. Cosmochim.Acta 47, 217-233.
Romanek,C.S., Chakraborty S., Li W., Beard B. and Johnson C.M., unpublished data.
Zhang F., Xu H., Konishi H. and Roden E.E. 2010 A relationship between d104 value and composition in the calcite-disordered dolomite solid-solution series. Amer. Mineral. 95, 1650-1656.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Collaborator
Clark Johnson
Collaborator
Nicholas Levitt
Collaborator
Weiqiang Li
Collaborator
-
RELATED OBJECTIVES:
Objective 7.1
Biosignatures to be sought in Solar System materials