2015 Annual Science Report
 University of Wisconsin
Reporting | JAN 2015 – DEC 2015
University of Wisconsin
Reporting | JAN 2015 – DEC 2015
Project 2A: Application of the 13C-18O Clumped Isotope Thermometer to the Ancient Earth
Project Summary
Application of the clumped isotope thermometer in carbonates, which is based on preferential bonding between 13C and 18O, offers the possibility of testing controversial proposals based on conventional stable isotope thermometry that the early Earth’s oceans were hot. In this project, we report on the results of a study of clumped isotope thermometry of the Neoarchean Campbellrand carbonate platform of South Africa. This platform is the best preserved Archean carbonate sequence known and was subjected to only very low grades of metamorphism, and hence offers the best opportunity to determine seawater temperature for the late Archean oceans. Comparison of clumped isotope temperatures retrieved from co-existing calcite and dolomite confirm that resetting of clumped isotope temperatures occurs at different rates for these minerals, where dolomite is closest to preserving primary temperatures. Despite slower re-equilibration rates for dolomite, however, the fact that the Campbellrand platform was buried at temperatures up to 170 oC for ~2 b.y., prevents use of clumped isotope thermometry from preserving Archean seawater temperatures. This would likely not be the case for carbonates on Mars, where early Mars carbonates would have had a relatively low temperature thermal history, suggesting that clumped isotope thermometry for Mars carbonates is a promising approach for determining ancient surface temperatures on Mars.
Project Progress
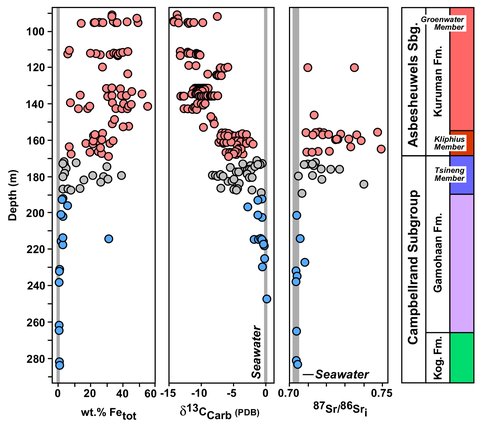
The Campbellrand-Malmani carbonate platform is located on the western margin of the Kaapvaal Craton of South Africa. The platform records a marine transgression that records shallow-water stromatolitic carbonate deposition in the lower parts of the platform, overlain by deep-water, iron-rich carbonate deposition (Fig. 1). This is accompanied by a transition from seawater C and Sr isotope compositions in the low-Fe carbonates of the Gamohaan Formation to compositions that do not reflect seawater in the overlying Fe-rich Kuruman Formation, indicating microbial diagenetic processes (Johnson et al., 2013).
In this study, we focused on shallow-water stromatolitic carbonates, both primary calcite and very early diagenetic dolomite from the Gamohaan Formation. Dolomitization is found in granular carbonate that represents detrital infill between stromatolitic mounds (Fig. 2), and therefore represents very early dolomitization. The goal was to evaluate the clumped isotope thermometer in terms of thermal history of the Gamohaan Formation as a function of mineralogy. Clumped isotope thermometry uses the preferential bonding between rare stable isotopes (e.g., 13C to 18O) in multiply-substituted isotopologues, which, in carbonates, is temperature dependent, and hence offers an advantage over traditional stable isotope thermometry in that knowledge of the isotopic composition of the fluid is not required.
Sumner and Beukes (2006) have estimated platform burial by thermal subsidence to be from 0.8km/100my to 4.9km/300my between ~2.7 and ~2.5 Ga. The maximum burial temperature of the platform has been estimated by Miyano and Beukes (1984) according to metamorphic mineral assemblages in the overlying Kuruman Iron Formation to be ~ 110-170 °C. Uplift and exhumation rates for the Kaapvaal Craton range from 4.4m/my to 800m/my (Bierman et al., 2014; Braun et al., 2014; Roberts and White, 2010; Flowers and Schoene, 2010). Braun et al. (2014) inferred uplift of the western Kaapvaal craton between 75 and 60 Ma at an average uplift rate of ~100m/my. Dauteuil et al. (2015) propose more recent uplift from ~30Ma to present.
Although the Campbellrand-Malmani sequence has experienced the lowest grade thermal history of any Archean carbonate platform, and hence is the best candidate for determining the temperature of the Late Archean oceans, it is possible that clumped isotope thermometry may not recover the primary formation temperatures. The objective of this study is to explore how the burial and exhumation history of the platform affected clumped isotope thermometry, using the solid-state reaction-diffusion based model of Stolper and Eiler (2015). The model of Stolper and Eiler presents kinetic data for calcite, and previous work suggests that dolomite will re-equilibrate more sluggishly, and the approach taken here is to compare clumped isotope thermometry in calcite and dolomite from the same samples. On-going efforts to determine the kinetics of dolomite by John Eiler’s group will provide firm constraints on the modeling, and in this report a variety of exchange kinetics are presented as a placeholder.
Clumped isotope temperatures (13C-18O) were measured from outcrop samples, including primary calcite and dolomite, as well as secondary hydrothermal carbonate, and young weathering carbonate. In addition, drill cores taken from the region were studied for comparison. Except for the young weathering carbonate, which returned a clumped isotope temperature of ~25 oC, calcite produced consistently high temperatures of ~130-160 oC. Dolomite consistently yielded lower temperatures of ~70-110 oC.
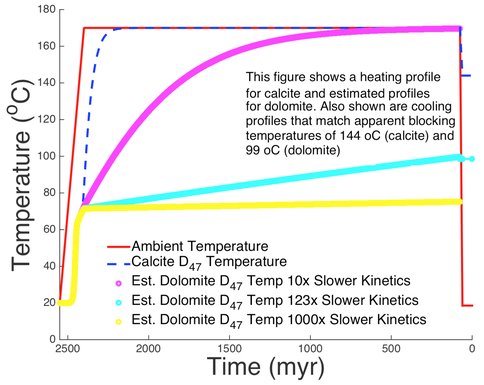
In Figure 3 we illustrate possible temperature-time histories that could reproduce the measured clumped isotope temperatures for calcite and dolomite using the model of Stolper and Eiler (2015). Calculations for calcite are the most robust as the clumped isotope kinetics have been published. Using the results of Sumner and Beukes (2006) and Miyano and Beukes (1984), we assume burial from 2.55 to 2.40 Ga from ambient conditions to a peak temperature of 170 oC. Under such conditions, calcite quickly attains the maximum temperature for clumped isotopes. Although some workers have argued for peak metamorphic conditions at ~2 Ga (e.g., Duane et al., 2004), the rapid equilibration of calcite would not change the results. Exhumation of the platform was modeled as occurring either between 75 and 60 Ma (Braun et al., 2014) or between 30 Ma and present (Dauteuil et al., 2015), which would produce calcite clumped isotope temperatures of 151 oC and 148 oC, respectively. These results indicate that for geologically reasonable exhumation rates, the final calcite clumped isotope temperature is ~20 oC cooler than peak conditions. That these results are in line with the maximum metamorphic temperatures estimated by Miyano and Beukes (1984) is highly encouraging. It is important to note that there is no evidence that peak metamorphic temperatures could have exceeded 200 oC, contrary to the inferences by Waldbauer et al. (2009) based on thermal maturity of organic matter.
For dolomite, we assume that only the long-term kinetic rate constant of Stolper and Eiler (2015) is different than calcite, and we illustrate three cases where dolomite kinetics are slower than calcite. If the long-term rate constant is 10 times slower than calcite, dolomite will eventually reach peak temperatures, and exhumation will lock in this temperature, producing clumped isotope temperatures for dolomite that are higher than those of calcite, which is clearly not the case. In order to match the average dolomite temperatures, the long-term rate constant for dolomite must be 123 times slower than calcite. If the long-term rate constant for dolomite is 1000 times slower, then lower temperatures for dolomite will be produced, ~70 oC, which lies on the low end of what was measured. These results will be improved once the kinetics of dolomite exchange and equilibration are known.
The models shown in Figure 3 make it clear that under no conditions can clumped isotope temperatures for calcite be used to infer seawater temperatures, even in the extremely low metamorphic grade of the Campbellrand-Malmani carbonate platform. It may be possible to place some general constraints using dolomite once the kinetics are known. Although it seems at this time that it is unlikely that original seawater temperatures may be inferred from this suite, it is impressive that the results paint a consistent story relative to what is known about the burial, metamorphic, and exhumation history of the platform. Moreover, this work demonstrates the value of clumped isotope thermometry in the search for biomarkers where an independent assessment of thermal maturity is needed for determining new drilling sites. In addition, for early carbonates on Mars, which likely saw much lower thermal histories than the Campbellrand-Malmani carbonate platform, dolomite would be highly likely to record carbonate formation temperatures, and even calcite might retain near-primary temperatures.
References Cited
Bierman et al. (2014) A cosmogenic view of erosion, relief generation, and the age of faulting in southern Africa. GSA Today, 24, 9, 4-10.
Braun, J; Guillocheau, F; Robin, C; Baby, G; Jelsma, H (2014) Rapid erosion of the Southern African Plateau as it climbs over a mantle superswell. J. Geophys. Res. Solid Earth 119, 6093-6112.
Dauteuil, O; Bessin, P; Guillocheau, F (2015) Topographic growth around the Orange River valley, southern Africa: A Cenozoic record of crustal deformation and climatic change. Geomorphology 233, 5‐19.
Duane, MJ; Kruger, FJ; Turner, AM, Whitelaw, HT; Coetzee, H; Verhagen, BT (2004) The timing and isotopic character of regional hydrothermal alteration and associated epigenetic mineralization in the western sector of the Kaapvaal Craton (South Africa). J. Afr. Earth Sci. 38, 461-476.
Flowers RM and Schoene B (2010) (U-‐Th)/He thermochronometry constraints on unroofing of the eastern Kaapvaal craton and significance for uplift of the southern African Plateau. Geology 38, 9, 827-830.
Johnson, CM; Ludois, JM; Beard, BL; Beukes, NJ; Heimann, A (2013) Iron formation carbonates: Paleoceanographic proxy or recorder of microbial diagenesis? Geology 41, 1147-1150.
Miyano, T; Beukes, NJ (1984) Phase Relations of Stilpnomelane, Ferri-annite, and Riebeckite in Very Low-Grade Metamorphosed Iron-Formations. Trans. Geol. Soc. S. Africa 87, 111-124.
Roberts, GG; White, N (2010) Estimating uplift rate histories from river profiles using African examples. J. Geophys. Res. 115, B02406.
Stolper, D. A. and J. M. Eiler. (2015) The Kinetics of Solid-State Isotope-Exchange Reactions for Clumped Isotopes: A Study of Inorganic Calcites and Apatites from Natural and Experimental Samples. Am. J. Sci., 315, 363-411.
Sumner and Beukes (2006) Sequence Stratigraphic Development of the Neoarchean Transvaal carbonate platform, Kaapvaal Craton, South Africa, Geol. Soc. S. Africa, 109, 11-22.
Waldbauer, JR; Sherman, LS; Sumner, DY; Summons, RE (2009) Late Archean molecular fossils from the Transvaal Supergroup record the antiquity of microbial diversity and aerobiosis. Precambrian Research 169, 28‐47.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Co-Investigator
John Eiler
Co-Investigator
Nicholas Levitt
Collaborator
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 5.2
Co-evolution of microbial communities
Objective 7.1
Biosignatures to be sought in Solar System materials