2015 Annual Science Report
 University of Wisconsin
Reporting | JAN 2015 – DEC 2015
University of Wisconsin
Reporting | JAN 2015 – DEC 2015
Project 1F: Study of Modern Fe-Mn Nodules in Green Bay Sediments as Analog to the Fe-Nodules and Fe-Oxyhydroxide Minerals on Mars
Project Summary
Fe-oxide nodules and concretions are common in terrestrial sedimentary rocks and also occur in Martial sediments. The precursors of the hematite are nano-phases of Fe-oxyhydroxides. Modern Fe-Mn nodules from Green Bay sediments were investigated by in-situ XRD, scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), Z-contrast imaging, and ab-initio calculations using the density functional theory (DFT) method. Nano-phase minerals for hosting trace elements of As, P, Ba, Co, Ni, and Zn have been identified. Structural sites of the trace elements and their incorporation mechanisms are also proposed. The Fe-Mn nodules can be used as analog for understanding the Fe-nodules and Fe-oxyhydroxide minerals on Mars.
Project Progress
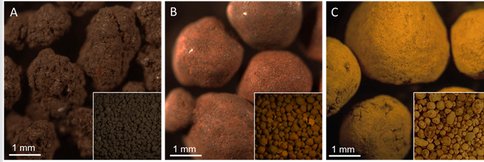
Green Bay nodules can be divided into three types: Mn-rich nodules, Fe-Mn nodules, and Fe-rich nodules (Fig. 1). All three types of nodules are nanoporous with dominated pore sizes of ~1.7 nm to ~10 nm. Mn-rich nodules are dominated by todorokite, birnessite, and buserite. Fe-rich nodules consist of feroxyhyte, goethite, 2-line ferrihydrite, and an FeOOH nano-phase with guyanaite structure. Most of the nodules have a concretionary structure of alternating Mn- and Fe-rich layers. The nodules commonly have cores of reddish feldspar containing hematite micro-crystals. Other core minerals are goethite, cristobalite, tridymite, hercynite, coal, chlorite-bearing rock fragments, and hematite-bearing quartz grains. The hexagonal or hexagonal-like structures of hematite micro-crystals and clay minerals in core minerals can serve as nucleation sites for Mn-oxides and Fe-oxyhydroxides and. For Mn-oxides, XRD patterns indicate transformation from birnessite to todorokite after precipitation. TEM-EDS spectra indicate that todorokite is associated with Ba, Co, Ni, and Zn, birnessite with Ca and Na, and buserite with Ca.
For Fe-oxyhydroxides, HRTEM and Z-contrast images show FeOOH nano-domains with guyanaite structure intergrown with goethite. The nano-phase is a precursor to goethite, and is assigned “proto-goethite” for this nano-phase. Density functional theory (DFT) calculation indicates that goethite is slightly more stable than proto-goethite. Overall, it is proposed that ordering between Fe and vacancies in octahedral sites resulted in transformation from feroxyhyte to goethite through an intermediate structure of proto-goethite. Combining Z-contrast images and TEM-EDS reveal that the arsenate AsO4 tetrahedron may be preferentially retained at the proto-goethite surface through tridentate attachment. This can also explain P, Si, and S associated with FeOOH nano-crystals. The observed alternating Fe/Mn layers in the FFN might be formed through oscillatory changes of redox conditions at the sediment-water interface.
Our results show that TEM observations, Z-contrast imaging, and ab initio calculations provided a powerful method to study crystal structure and chemistry of poorly crystallized Fe/Mn nano-minerals. Z-contrast images directly show the atomic positions and their interface between neighboring phases and their relationship. Ab-initio calculation reveals that goethite is the most stable phase for the FeOOH polymorphs. In addition, Z-contrast images show arsenic tetrahedra are preferentially adsorbed on the (001) surface of proto-goethite. The single chain of proto-goethite provides more structurally favorable AsO4- affinity through the tridentate complex than the bidentate complex on goethite, and consequently poorly crystalline outer layers can contain the more arsenic.
TEM and associated techniques are useful tools to identify individual nanoparticles and to study the chemistry of nanoparticles. Previous studies only revealed correlations between Fe/Mn ratios and trace elements. However, our method can provide the detailed chemical composition of individual Fe/Mn nano-phases, not just bulk. From the TEM-EDS spectra, we can gain detailed average chemical formula of todorokite, Ba2+0.28(Zn+20.13, Co3+0.05, Ni2+0.02)(Mn4+5.01, Mn3+0.54, Fe3+0.38 Co3+0.05, Ni2+0.02)O12•nH2O, birnessite, Na+0.14Ca2+0.19 (Mn4+1.48, Mn3+0.52)O4 •nH2O, and Ca-buserite, Ca2+0.27(Mn4+1.46, Mn3+0.54)O4 •nH2O.
Interestingly, Green Bay Fe-Mn nodules share similar physical characteristic with Fe-oxide spherules “blueberries” from Meridiani Planum (Chan et al., 2012; McLennan et al., 2005). Mars blueberries have sizes about 5mm in diameter, similar to the Green Bay FFN (Potter et al., 2011). Occasional doublets or triplets’ occur in both the nodules and Mars spherules (Calvin et al., 2008). Internal structures are also documented on Mars (Calvin et al., 2008). These characteristics indicate a general formation via groundwater diagenesis (McLennan et al., 2005; Potter et al., 2011). A main difference between the two units is mineralogy. Ferrihydrite, goethite, and hematite are present in the Martian spherules, whereas Green Bay nodules do not contain hematite (Calvin et al., 2008; Chan et al., 2012; Potter et al., 2011). However, the ferrihydrite and goethite can be dehydrated to hematite when those phases are exposed in dry environment (Glotch et al., 2004).
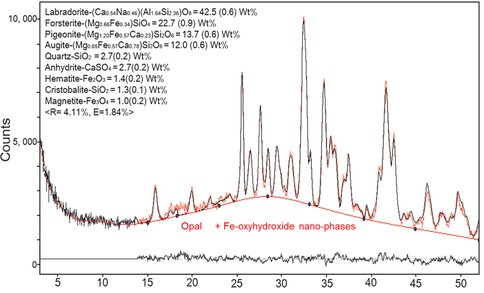
The CheMin XRD patterns of Rocknest, John Klein, and Cumberland in Mars were reported in previous studies (Bish et al., 2013; Blake et al., 2013; Vaniman et al., 2014). We found that an additional cristobalite phase might be in the sample (Fig. 2). Some Lunar Mare basalts constitute up to 5 vol.% cristobalite (Chao et al., 1970).
The XRD patterns and the chemical composition of the amorphous component of Rocknest, John Klein, and Cumberland, Mars suggest that Fe nano-phases are present and abundant (Bish et al., 2013; Blake et al., 2013; Vaniman et al., 2014). It is evidenced in the XRD patterns by the elevated background between 14º (7.32 Å) to 53º (2.00 Å). XRD patterns of Green Bay samples have the background between 5.1 Å and 2.0 Å, indicated by Fe-oxyhydroxide nano-phases. Thus, it is expected to have similar Fe-oxyhydroxide nano-phases in the two systems.
Furthermore, the nano-phase Fe-oxyhydroxides can absorb SiO4, PO4, and SO4 strongly in tridentate complex. A chemical composition of amorphous component of Rocknest reported 37.20 wt% SiO2, 23.14 wt% FeO+Fe2O3, 2.09 wt% P2O5, and 11.01 wt% SO3 (Blake et al., 2013). Our TEM-EDS result of the Fe-oxyhydroxides (especially in proto-goethite rich area) also shows high concentration of these components (18.8 wt% SiO2 and 2.4 wt% P2O5).
References
Bish, D.L., Blake, D.F., Vaniman, D.T., Chipera, S.J., Morris, R.V., Ming, D.W., Treiman, A.H., Sarrazin, P., Morrison, S.M., Downs, R.T., Achilles, C.N., Yen, A.S., Bristow, T.F., Crisp, J.A., Morookian, J.M., Farmer, J.D., Rampe, E.B., Stolper, E.M., Spanovich, N., and Team, M.S. (2013) X-ray Diffraction Results from Mars Science Laboratory: Mineralogy of Rocknest at Gale Crater. Science, 341(6153).
Bish, D.L., and Post, J.E. (1989) Thermal-Behavior of Complex, Tunnel-Structure Manganese Oxides. American Mineralogist, 74(1-2), 177-186.
Blake, D.F., Morris, R.V., Kocurek, G., Morrison, S.M., Downs, R.T., Bish, D., Ming, D.W., Edgett, K.S., Rubin, D., Goetz, W., Madsen, M.B., Sullivan, R., Gellert, R., Campbell, I., Treiman, A.H., McLennan, S.M., Yen, A.S., Grotzinger, J., Vaniman, D.T., Chipera, S.J., Achilles, C.N., Rampe, E.B., Sumner, D., Meslin, P.Y., Maurice, S., Forni, O., Gasnault, O., Fisk, M., Schmidt, M., Mahaffy, P., Leshin, L.A., Glavin, D., Steele, A., Freissinet, C., Navarro-Gonzalez, R., Yingst, R.A., Kah, L.C., Bridges, N., Lewis, K.W., Bristow, T.F., Farmer, J.D., Crisp, J.A., Stolper, E.M., Marais, D.J.D., Sarrazin, P., and Team, M.S. (2013) Curiosity at Gale Crater, Mars: Characterization and Analysis of the Rocknest Sand Shadow. Science, 341(6153).
Calvin, W.M., Shoffner, J.D., Johnson, J.R., Knoll, A.H., Pocock, J.M., Squyres, S.W., Weitz, C.M., Arvidson, R.E., Bell, J.F., Christensen, P.R., de Souza, P.A., Farrand, W.H., Glotch, T.D., Herkenhoff, K.E., Jolliff, B.L., Knudson, A.T., McLennan, S.M., Rogers, A.D., and Thompson, S.D. (2008) Hematite spherules at Meridiani: Results from MI, Mini-TES, and Pancam. Journal of Geophysical Research-Planets, 113(E12).
Chan, M.A., Potter, S.L., Bowen, B.B., Parry, W.T., Barge, L.M., Seiler, W., Petersen, E.U., and Bowman, J.R. (2012) Characteristics of Terrestrial Ferric Oxide Concretions and Implications for Mars. Sedimentary Geology of Mars, 102, 253-270.
Chao, E.C.T., James, O.B., Minkin, J.A., Boreman, J.A., Jackson, E.D., and Raleigh, C.B. (1970) Petrology of Unshocked Crystalline Rocks and Shock Effects in Lunar Rocks and Minerals. Science, 167(3918), 644-&.
McLennan, S.M., Bell, J.F., Calvin, W.M., Christensen, P.R., Clark, B.C., de Souza, P.A., Farmer, J., Farrand, W.H., Fike, D.A., Gellert, R., Ghosh, A., Glotch, T.D., Grotzinger, J.P., Hahn, B., Herkenhoff, K.E., Hurowitz, J.A., Johnson, J.R., Johnson, S.S., Jolliff, B., Klingelhofer, G., Knoll, A.H., Learner, Z., Malin, M.C., McSween, H.Y., Pocock, J., Ruff, S.W., Soderblom, L.A., Squyres, S.W., Tosca, N.J., Watters, W.A., Wyatt, M.B., and Yen, A. (2005) Provenance and diagenesis of the evaporite-bearing Burns formation, Meridiani Planum, Mars. Earth and Planetary Science Letters, 240(1), 95-121.
Potter, S.L., Chan, M.A., Petersen, E.U., Dyar, M.D., and Sklute, E. (2011) Characterization of Navajo Sandstone concretions: Mars comparison and criteria for distinguishing diagenetic origins. Earth and Planetary Science Letters, 301(3-4), 444-456.
Vaniman, D.T., Bish, D.L., Ming, D.W., Bristow, T.F., Morris, R.V., Blake, D.F., Chipera, S.J., Morrison, S.M., Treiman, A.H., Rampe, E.B., Rice, M., Achilles, C.N., Grotzinger, J.P., McLennan, S.M., Williams, J., Bell, J.F., Newsom, H.E., Downs, R.T., Maurice, S., Sarrazin, P., Yen, A.S., Morookian, J.M., Farmer, J.D., Stack, K., Milliken, R.E., Ehlmann, B.L., Sumner, D.Y., Berger, G., Crisp, J.A., Hurowitz, J.A., Anderson, R., Des Marais, D.J., Stolper, E.M., Edgett, K.S., Gupta, S., Spanovich, N., and Team, M.S. (2014) Mineralogy of a Mudstone at Yellowknife Bay, Gale Crater, Mars. Science, 343(6169).
Publications
-
Schindler, M. (2016). Study on nanophase iron oxyhydroxides in freshwater ferromanganese nodules from Green Bay, Lake Michigan. American Mineralogist, 101(9), 1927–1927. doi:10.2138/am-2016-5893
-
Xu, H., Shen, Z., & Konishi, H. (2014). Si-magnetite nano-precipitates in silician magnetite from banded iron formation: Z-contrast imaging and ab initio study. American Mineralogist, 99(11-12), 2196–2202. doi:10.2138/am-2014-4964
-
Xu, H., Shen, Z., & Konishi, H. (2015). Natural occurrence of monoclinic Fe3S4 nano-precipitates in pyrrhotite from the Sudbury ore deposit: a Z-contrast imaging and density functional theory study. Mineralogical Magazine, 79(2), 377–385. doi:10.1180/minmag.2015.079.2.15
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Zhizhang Shen
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 7.1
Biosignatures to be sought in Solar System materials