2015 Annual Science Report
 University of Wisconsin
Reporting | JAN 2015 – DEC 2015
University of Wisconsin
Reporting | JAN 2015 – DEC 2015
Project 1A: Ground Control Experiments for the OREOcube Mission
Project Summary
The OREOcube (ORganics Exposure in Orbit cube) experiment has been designed to study the effects of solar and cosmic radiation on astrobiology relevant organics when associated with mineral surfaces. The goal of this project is to investigate the (photo)chemical evolution and processing of organic matter in simulated proto-planetary and planetary microenvironments in the laboratory and in space. Testing the photostabitity and evolution of organics in actual space environments on the International Space Station (ISS) provides a powerful tool to study the combined impact of several relevant parameters (e.g., ultraviolet and comic radiation, microgravity) simultaneously. The OREOcube capability to measure in-situ changes of samples exposed to space radiation using an UV-visible-NIR spectrometer as a function of time in Low Earth Orbit will confer significant advantages relative to more basic ISS exposure facilities for which sample measurements are made on Earth prior to and at the end of a space mission. The innovative aspect of this OREOcube experiments is the capability of in-situ monitoring of flight samples, which has not yet been achieved on the ISS. A comparison of measurements performed with OREOcube with recent LEO data (EXPOSE-E, EXPOSE-R, O/OREOS Sat) and ground-based laboratory data will reveal how those combined data from different exposure facilities can be effectively used to investigate the evolution of organic matter in space.
Project Progress
In collaboration with NASA Ames Research Center, Leiden University, and the SETI Institute, pre-flight experiments using sets of astrobiologically important organic samples in combination with mineral substrates have been performed under conditions that simulate anticipated conditions on an International Space Station external platform. In an OREOcube experiment, an adsorbate interface is defined by depositing organic samples as thin films onto solid substrates. Samples are housed in hermetically sealed reaction cells containing an internal test environment that allows control of headspace gases including the partial pressure of water vapor, see Figure 1. This provides a controlled method to examine organic samples and inorganic surface interactions.
Tested inorganic substrates include hematite, magnetite, chromium oxide, titanium dioxide, nickel oxide, silica and alumina in combination with four classes of organics, polycyclic aromatic hydrocarbon (PAH), amino acid, metalloporphyrin, and quinone. The gathered data will allow us to select the most suitable and scientifically relevant organic-inorganic thin films combinations to be exposed on the ISS. Pre-flight ground-based research on the photochemistry of anthrarufin and iron tetraphenylporphyrin show that these compounds interact strongly with iron oxides in terms of electronic structure resulting in altered spectral features and overall absorptivity relative to other inorganic substrates, see Figure 2.
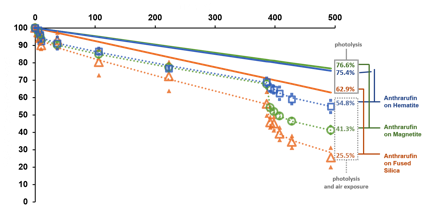
Our experiments show that the photolytic degradation on iron oxides is slower compared to silica and other substrates, which may result in improved preservation potential for these compounds.
Publications
-
Cook, A. M., Mattioda, A. L., Quinn, R. C., Ricco, A. J., Ehrenfreund, P., Bramall, N. E., … Walker, R. (2014). SEVO ON THE GROUND: DESIGN OF A LABORATORY SOLAR SIMULATION IN SUPPORT OF THE O/OREOS MISSION. The Astrophysical Journal Supplement Series, 210(2), 15. doi:10.1088/0067-0049/210/2/15
-
Cook, A. M., Mattioda, A. L., Ricco, A. J., Quinn, R. C., Elsaesser, A., Ehrenfreund, P., … Hoffmann, S. V. (2014). The Organism/Organic Exposure to Orbital Stresses (O/OREOS) Satellite: Radiation Exposure in Low-Earth Orbit and Supporting Laboratory Studies of Iron Tetraphenylporphyrin Chloride. Astrobiology, 14(2), 87–101. doi:10.1089/ast.2013.0998
-
Demets, R., Bertrand, M., Bolkhovitinov, A., Bryson, K., Colas, C., Cottin, H., … Schuster, M. (2014). Window contamination on Expose-R. International Journal of Astrobiology, 14(01), 33–45. doi:10.1017/s1473550414000536
-
Ehrenfreund, P., Ricco, A. J., Squires, D., Kitts, C., Agasid, E., Bramall, N., … Young, A. (2014). The O/OREOS mission—Astrobiology in low Earth orbit. Acta Astronautica, 93, 501–508. doi:10.1016/j.actaastro.2012.09.009
-
Elsaesser, A., Quinn, R. C., Ehrenfreund, P., Mattioda, A. L., Ricco, A. J., Alonzo, J., … Santos, O. (2014). Organics Exposure in Orbit (OREOcube): A Next-Generation Space Exposure Platform. Langmuir, 30(44), 13217–13227. doi:10.1021/la501203g
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Richard Quinn
Co-Investigator
Kathie Bryson
Collaborator
Andreas Elsässer
Collaborator
Andrew Mattioda
Collaborator
Antonio J. Ricco
Collaborator
Farid Salama
Collaborator
-
RELATED OBJECTIVES:
Objective 3.1
Sources of prebiotic materials and catalysts