2015 Annual Science Report
 University of Southern California
Reporting | JAN 2015 – DEC 2015
University of Southern California
Reporting | JAN 2015 – DEC 2015
Life Underground
Project Summary
Our multi-disciplinary team from the University of Southern California, California Institute of Technology, Jet Propulsion Lab, Desert Research Institute, Rensselaer Polytechnic Institute, and Northwestern University is developing and employing field, laboratory, and modeling approaches aimed at detecting and characterizing microbial life in the subsurface—the intraterrestrials. We posit that if life exists, or ever existed, on Mars or other planetary body in our solar system, evidence thereof would most likely be found in the subsurface. This study takes advantage of unique opportunities to explore the subsurface ecosystems on Earth through boreholes, mine shafts, sediment coring, marine vents and seeps, and deeply-sourced springs. Access to the subsurface—both continental and marine—and broad characterization of the rocks, fluids, and microbial inhabitants is central to this study. Our focused research themes require subsurface samples for laboratory and in situ experiments. Specifically, we are carrying out in situ life detection, culturing and isolation of heretofore unknown intraterrestrial archaea and bacteria using numerous novel and traditional techniques, and incorporating new and existing data into regional and global metabolic energy models.
Project Progress
The centerpiece of our research effort is a set of promising and geologically-representative deep subsurface sites on Earth, both continental and marine. These sites provide samples and opportunities around which we are conducting several comprehensive, interdisciplinary, coordinated, and complementary research efforts. Here, we provide brief summaries of the main accomplishments during this reporting period for four major research themes: (A) access to the subsurface and broad characterization; (B) in-situ life detection and characterization, including mission relevance; (C) guided cultivation of intraterrestrials; and (D) energy flow and metabolic modeling.
A. Access to the Subsurface and Broad Characterization.
In 2015, the Life Underground project built upon our 2014 and prior work at the Sanford Underground Research Facility (SURF) in South Dakota and greatly expanded our work at a borehole site near Death Valley, CA; we also continued ongoing work at an ophiolite site in northern California called ‘the Cedars’. In the marine realm, work continued on samples acquired from the Shimokita Peninsula (Japan) in the northwestern Pacific (IODP Drilling Expedition 337, see Section 3B), and collaborations continued with the NSF-funded Science and Technology Center for Dark Energy Biosphere Investigations (C-DEBI), which focuses on the marine subseafloor biosphere (see Section 9). For all sites—continental and marine—collections of standardized samples and metadata continued, including: temperature, pH, oxidation-reduction potential, dissolved oxygen, total dissolved solids, aqueous and nutrient chemistry analysis, metal speciation, dissolved gas compositional and isotopic characterizations (dC and d2H), sulfur and water isotopes (e.g. d34S and d2H/d18O), radiocarbon, organic constituents, electron microscopy, planktonic cell abundance, cultivations, single cell genomics, filters for DNA and lipids, and bulk water for microbial cultivation. We have shifted in focus from our early strategy of preliminary site characterization to one of time series sampling and the establishment of long-term monitoring at the SURF, BLM-1, and the Cedars. (See also Section 7 for further description of our field sites.)
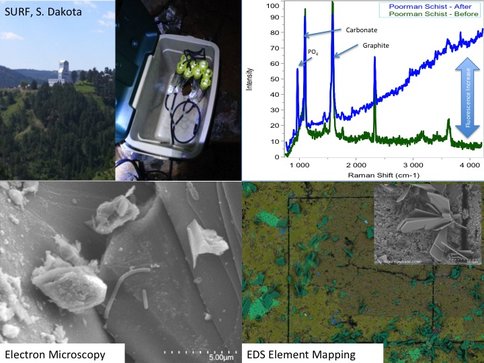
SURF: Several projects were continued or initiated in 2015, expanding from basic site characterization to targeted analysis. This work is facilitated by the fact that many Life Underground team members are now safety-certified to work at SURF. A keystone activity was our participation in an underground drilling project on the 4850 level, (1,478 m depth where we were allowed to take 3.2 m of the ~168 m core (LBNE-14-3), drilled by the facility for other purposes. This core was selected for its remoteness from historic mining and proximity to potentially water-bearing Tertiary dykes. Core samples were processed underground for preservation of microbial DNA (freezing), cultivation, and deep UV scanning. Analysis of preliminary DNA extracts from the core material and control drilling fluid samples revealed the presence of key subsurface lithotrophic phyla. Full characterization of these samples will be undertaken in 2016. Another highlight of 2015 was the completion and harvesting (May 2015) of our in situ electrode-assisted cultivation reactor also on the 4850 level. Here, subsurface microbes were cultivated directly from legacy borehole fluids onto electrodes poised at 4 different potentials. Cultivation and molecular studies are now underway from these samples and preliminary results suggest successful microbial selection based on electrochemical gradients. The ongoing phase of work at SURF will involve the development of a distributed, kilometer-scale, 3D microbial observatory using select legacy holes from the surface to the 4850 level. Preliminary characterization (chemical, biological, physical, and logistical) of candidate sites was completed in December of 2015 and significant progress is expected in the coming year on the design and instillation of monitoring stations at the six finalist sites.
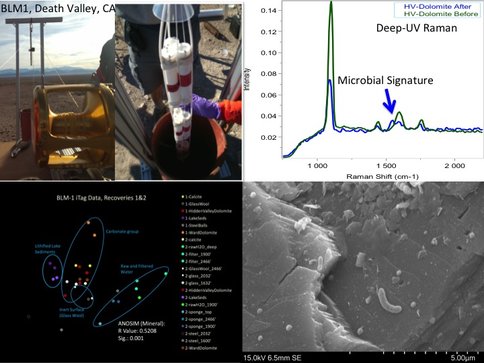
Great Basin: Extensive work was completed in 2015 at the “Tier-1” borehole, known as BLM-1 near Death Valley, CA. Beginning in November of 2014, major activities revolved around four sequential downhole incubations (three, six, and three months and one ongoing) of substrate cartridges within the casing and in a zone of open hole completion below the casing. Incubated materials included dolomitic and tuffaceous rock chips (commercially available and from the hole itself), sponges, mineral coupons, glass wool, and steel casing material. In August, the hole was logged, fluids sampled, and downhole tools deployed, including a caliper, thermal heat flow meter (TFM), Idronaut Chem Tool (Temp, pH, conductivity, ORP, and dO2) and electronically-actuated discrete samplers (1L and 5L). Caliper logging showed an unscreened steel casing from surface to 749.8 m with open hole below to an obstruction at 753.8 m, below which access is prevented. TFM analysis failed to detect any evidence of advective flow anywhere within the hole. Conditions within the hole were suboxic with a maximum temperature of ~57°C below the casing (down to 749.8 m). Aside from the substrates incubated within the hole, water samples were collected from within the casing at 609.6 m and in the uncased hole at 751.3 m. All materials incubated and retrieved from the hole are being assessed for colonial microbial community composition. Additionally, post-incubation materials are being microbially and mineralogically characterized via electron microscopy and spectroscopy (see Section 3B), analyzed for protein content, and used as inocula for multiple lab-based culturing endeavors. Water samples were collected at pressure during logging and are being analyzed for all of the chemical and biological analyses noted above. Samples are also being processed for radiocarbon analysis. The ‘baseline’ state incubations already completed set the stage for the next phase of experiments, which will focus on in situ characterization of microbial activity and monitoring of community composition changes over time in response to various stimuli. Also in 2016, we anticipate deploying a version of the BLM-1 experiment in at least one additional hole, most likely at one located >100 km up-gradient in the same regional flow system in Tertiary volcanics on the Nevada National Security Site (NNSS).
The Cedars: This site in northern California is part of an ophiolite complex characterized by peridotite serpentinization that yields fluids of very high pH, low redox potential, and low ionic strength. The Cedars has been under continuous study by Co-I Nealson and colleagues for a number of years. In 2015, transcriptomic studies of the alkaliphilic isolate Serpentinomonas sp. were begun, efforts to isolate subsurface organisms from the site yielded enrichments of Firmicutes from the subsurface, and an electrochemically-active microorganism, isolated from the site, was characterized.
B. In-situ Life Detection and Characterization, and Mission Relevance.
In this theme, we aim to detect and characterize in situ life based on spatial distribution of organics (e.g., microorganisms), minerals, elements and isotopes, in the context of textural features, from the macro to the micro scale. The team uses a number of optical techniques covering the electromagnetic spectrum from the deep UV (<250 nm) to the IR; using an array of spectral phenomena such as absorption, Raman scattering, fluorescence, and reflectance, for noninvasive means of assessing macro to micro structure, organics, minerals, and elements. These methods help refine samples to smaller targets of interest for analysis by more “invasive” but chemically-specific analyses, including phylogenetic identification of microorganisms and community structure to elemental/isotopic analysis using techniques such as nanoSIMS.
Instrument calibration with cultured archaea and bacteria. Detecting microbes on surfaces using the Deep-UV fluorescence/Raman instruments at JPL has now been demonstrated, however it is not known how different stages of microbial growth (e.g., exponential vs. stationary phase) may alter these spectra. As many deep subsurface microorganisms are believed to be in a dormant or low activity state, it is critical to evaluate how these variables influence the spectral output measured by Deep-UV fluorescence/Raman. To better constrain this parameter, we have conducted a series of well-controlled lab experiments using a variety of bacterial and archaeal species collected at various stages of growth and analyzed them on the deep-UV fluorescence/Raman instruments in an attempt to discern the chemometric space that each microbe occupies. It is expected that the activity of a microbe influences spectral features since the response is associated to the production/concentration of aromatic amino acids (deep UV fluorescence/Raman), nucleic acids and lipids (deep UV Raman). The data are currently being analyzed and examined for potential overarching trends as well as the degree of variance in output signal for the different bacterial and archaeal strains.
In situ sample analysis and colonization experiments and development of the macro-to-micro life detection pipeline. To characterize the subsurface microbial assemblage and colonization patterns, we deployed several sets of defined mineral and colonization substrates (see Section 3A) for in situ incubations at both the SURF and BLM-1 field sites. We have recently recovered three suites of samples from BLM-1 and one from the 4850 level at SURF. These samples, specifically mineral coupons, are now being used to develop a macro-to-micro life detection and characterization pipeline. Over the last year, significant technical advancements have been made, allowing for a means to search for and target microbial and geochemically-informative regions at the centimeter scale and enabling samples to be moved between instruments for a finer scale, higher resolution analyses while maintaining spatial correlations at the micron-scale. We are currently in the process of co-registering these data sets. This has required modifications to tried-and-tested methods, sample workflow, as well as the writing of a Multi-INstrument Database (MIND), and new analysis software to handle data from various instruments over a series of spatial scales.
In 2014, we focused on developing the sample analysis process using a series of lab standards, samples incubated in the field operating as “windows” to the subsurface, and subsurface materials collected previously. In 2015, we expanded these analyses to include microbial colonization of incubated carbonate minerals and native lithologies from the SURF and BLM1 site, mapped at the cm-mm- and μm-scale. This analytical pipeline is continually being refined, but initial results demonstrate the uniqueness of the techniques applied in tandem. One technique for field and lab samples, deep UV Raman/fluorescence, has been under development for the past 18 years as a means to search for organics and habitable regions without the need for sample handling. This technique was selected for the upcoming Mars 2020 mission as the flight instrument SHERLOC (Scanning Habitable Environment with Raman and Luminescence for Organics and Chemicals) where Co-I’s/Collaborators of the Life Underground team are SHERLOC Deputy PI (R. Bhartia) and Co-I’s (K. Nealson and B. Ehlmann).
Integration of instrumentation aligned with the Mars 2020 payload and field testing. In a similar manner to the stated goals of Mars 2020, the Life Underground team is using deep UV Raman/fluorescence as a means to detect and map organics and microbes living in rock, such that we can better focus detailed mineralogical, structural, and elemental/isotopic techniques. Aligned with the Mars 2020 instrument payload, the Life Underground team is continuing efforts to combine multispectral, multispatial, and temporal methods to enable “correlative imaging” as a means to better understand the living subsurface communities. The deep UV instruments are being tested for both laboratory analyses and in situ use at the Life Underground field sites. The ability to co-register compatible suites of data collected on the same sample at small spatial scales is of broad value and interest to multiple NAI teams and as such, we have begun conversations with team members from Rock Powered Life and others to further extend the utility of these analytical imaging approaches.
The Life Underground team took advantage of a unique drilling opportunity at the SURF site. A newly-planned underground physics laboratory, the Long Baseline Neutrino Experiment required rock core samples be drilled on the 4850 level as part of a geotechnical assessment prior to construction of the underground cavern. Several members of the NAI team were on location at SURF as the cores were drilled and set up a field ready lab to perform onsite analyses of the freshly drilled material, including scanning of the cores with MOSAIC, the Deep UV- Fluorescence mapping instrument (a predecessor to the SHERLOC instrument described below). Data from these field scans continue to be analyzed. Sections of core were also preserved onsite and returned to USC and JPL for downstream analyses, including cryodrilling for DNA extraction and sequencing, microscopy, and higher resolution Deep UV scanning. The MOSAIC scans of the cores did not definitively detect microorganisms in the field, but the MOSAIC did show areas of the core that were contaminated from drilling. This allowed us to select minimally impacted core material for downstream analysis. MOSAIC scans showed that contamination was concentrated on the exterior of the cores and fracture surfaces exposed during the drilling operations and did not penetrate into the interior. At JPL and Caltech, we tested a new method for recovering genetic material from the interior of the (hard rock) cores while minimizing contamination from the core exterior. Intact frozen cores were cryogenically sampled using a modified drill apparatus. DNA from these samples and corresponding contaminant controls (e.g. drilling fluid) was extracted and the microbial diversity is currently being assessed with next generation iTAG sequencing. With this testing completed, a full set of sampling and extractions on additional archived cores is planned at regular intervals along the length of two cores (LBNE-14-3 and LBNE-14-4). The shallowest sample is from the tunnel interface (~2 m) with the final sample ~200 m into the undisturbed rock. This transect should allow us to determine microbial signatures that may be related to mining operations (i.e. the shallow sample) traversing into deeper regions of the rock.
In situ field deployments: SURF. Characterization of SURF continued this year with the installation of two long-term experiments plumbed directly into two boreholes. The first of these consists of a series of cartridges filled with crushed native minerals collected previously from the 4850 level (Figure 1). Along with the crushed minerals, several polished coupons of the same minerals were included. These mineral coupons were characterized prior to deployment with the Deep-UV Raman and Fluorescence instruments and electron microscopy with elemental analysis. These t=0 data are helping us to design and streamline operations and data management. These coupons represent ‘simplified’, mineral-only controls that we are using to verify the macro-to-micro analysis pipeline for all samples collected in the future. The SURF cartridges will be collected in March/April 2016 and will be processed though the entire analytical pipeline at JPL and Caltech (i.e. Photography, Deep-UV Fluorescence/Raman, FISH-Fluorescence Microscopy, Electron Microscopy and nanoSIMS). Efforts have been taken so that features on the mineral chips are spatially registered allowing for direct comparisons of features occurring pre and post incubation.
The initial set of cartridges was recovered from SURF in April 2015. DNA from these minerals has been extracted and sequenced. Analysis of the microbial communities is currently ongoing. The mineral coupons have been processed though a portion of the spectral pipeline (Deep-UV Raman/fluorescence and Electron Microscopy) (Figure 2). At the time of recovery of the first experiment, a second, similar cartridge experiment was deployed in another region of the mine on the 4850 level at a borehole flowing at ~6L/min at 30°C. This site will become a major site for our underground microbial observatory at SURF and as such, the cartridges recovered in October 2015 from this site, combined with the geochemistry collected in previous years, set the baseline for future studies by the NAI team.
In situ field deployments at BLM-1: Characterization and sampling of borehole BLM-1 has continued. In November 2014, we successfully deployed a large suite of mineral and inert substrate samples in the borehole. A sample string consisting of sponges and mixed mineral cartridges (Figure 2), similar to those deployed at SURF, were placed into the borehole and allowed to incubate until February 2015 (see section 3A). Polished coupons of the minerals will be subjected to the macro-to-micro sample pipeline (i.e. Spectroscopy→ Microscopy → NanoSIMS and molecular characterization). The substrate incubation experiments from February, August, and October, provided a unique sample suite to study the stability of the microbial assemblage over the course of a year from different depths in the cased and uncased portion of the well. Mineral colonization experiments also enabled assessment of in situ mineral colonization patterns associated with subsets of the microbial community (Figure 2) as well as visual information of the spatial colonization patterns, potential mineral alteration, and secondary mineral formation. For example, SEM and EDX analysis of the dolomite mineral coupons revealed the ingrowth of small, micron-scale, framboidal-like iron sulfides on the surfaces over the course of the in situ incubation. Notably, these framboids were often associated with a single cell or small group of cells and appeared to be unique to two independent dolomite samples and not observed on other minerals incubated in parallel in the same cartridge. Follow-up mineral incubations containing a variety of sulfide minerals were deployed in a subsequent field excursion to BLM-1, and the samples are currently being processed through our analytical pipeline.
Passive in situ colonization experiments such as those mentioned above are ideal for understanding the microbial community structure and potential mineralogical controls, but metabolic activity and growth from these early experiments can only be indirectly estimated by colonization patterns. In 2016, we are coordinating experiments using stable isotope amendments with in situ colonized samples from the BLM-1 borehole. In consultation with engineers at the Scripps Institution of Oceanography, we are developing a novel sampling device for recovery and transport of samples under pressure to the lab for direct isotope labeled substrate amendments.
In situ field deployments at the Cedars: Work at the deeply-sourced springs at ‘the Cedars’ also continued in 2015. In September, we installed passive culturing devices attempting to isolate some of the microbes in the GPS spring. During previous visits to the Cedars our efforts at the GPS site (the deepest sources water) were directed toward genomic and metagenomic sample collection. With the bulk of these analyses completed and published efforts could now turn to isolation and characterization of some of the culturable organisms within the spring. After an in-depth description of the study site (Morrill et al., 2013), a detailed analysis of the in situ microbial populations (Suzuki et al., 2013), new methods for population analysis using metatranscriptomics (Ishii et al., 2013a), and the detection of genes activated by changes in surface electrochemical charge (Ishii et al., 2013b), we finished some detailed studies of the physiology of bacteria isolated from the Cedars (Suzuki et al., 2014). In addition, we deployed and recovered mineral colonization experiments. The recovered mineral coupons were rescanned with the deep-UV Raman and fluorescence systems to map the extent of the microbial colonization. The DNA on the samples was then extracted and is currently undergoing sequencing to determine the microbial community structure. A suite of carbonate samples was also collected from ‘the Cedars’, spanning a range of ages (e.g., 1-2 years, 100’s of years and 1000’s of years old). These samples have been scanned with the Deep-UV instruments and maps of the minerals and entrained organics have been created. These data are currently being worked up.
Deep subseafloor sampling and stable isotope probing experiments: On the marine side of the Life Underground project, Caltech graduate students Trembath-Reichert, Case, and Marlow have been involved in sample collection and experimental set up for deep subseafloor sites. Trembath-Reichert sailed on the 2012 IODP Expedition 337 (Shimokita Coalbed) and worked with NAI collaborating partners from JAMSTEC in Japan (Inagaki and Moreno) to prepare stable isotope incubations (13C, 15N and D/H) from 4 different lithologies ranging from sandstone to coal. The chosen study area for Expedition 337 had a high probability to retrieve core from deeply buried coal beds of low thermal maturity, increasing the potential to find deep life by exploring an environment that could utilize coal deposits for carbon and potentially other essential nutrients. Geochemical analyses of progressive 13C enrichment in the DIC pool and CH4 production over the course of a 30-month incubation indicated slow but active microbial respiration in the >2 km coal bed samples with C1 carbon substrates. Samples from the deep coal bed were harvested by Trembath-Reichert and prepared in the ultraclean room facility in the JAMSTEC Kochi Core processing Center in Japan in collaboration with NAI collaborator Fumio Inagaki and Yuki Moreno in the summer of 2015. Cell concentrates from these samples were recovered and recently analyzed on the nanoSIMS 50L instrument at Caltech, demonstrating active assimilation of C1 compounds as well as providing some of the first approximations of growth rates through the enrichment of D/H in cells after incubation with deutrated water. Deep UV-Raman analysis of the coal from Shimokita is being used to map putative microbial features associated with the coal as well as used as a geothermometer of the maturity of the associated organics.
C. Guided Cultivation of Intraterrestrials.
Since the characterization of the subsurface biosphere is often challenging because many resident microbes appear ‘unculturable’ using traditional growth methods, we are focused on developing and harnessing new cultivation and enrichment techniques that go beyond these traditional limitations. Our approaches have been applied both in situ and ex situ to mimic the complex interactions and energetic gradients present in the NAI field sites, in order to reveal the full diversity of subsurface microorganisms. During this reporting period, our progress on the cultivation front was as follows:
Down-flow Hanging Sponge Reactors for investigating microbial communities in porous media. Graduate student Lily Momper in the Amend lab at USC constructed, tested, and operated down-flow hanging sponge (DHS) bioreactors under two conditions for enrichment of (i) Metal (Fe/Mn) reducing bacteria and (ii) Ammonia oxidizing Archaea, or Thaumarchaeota, under micro-oxic conditions (Amend Lab). The DHS technology was transferred to our team members (Amend, Orphan) from our JAMSTEC collaborator (Dr. Hiroyuki Imachi). These bioreactors were inoculated from our preliminary sampling projects at Nevares Deep Well 2 and SURF. Preliminary results indicated successful enrichment, as evidenced by biomass measurements, 16S rRNA analysis, and microscopy. From the Nevares Deep Well 2 enrichments, two novel bacteria have been isolated. One falls within the genus Thermincola, a group of chemolithoautotrophis known to reduce ferric iron with hydrogen as an electron donor using carbon dioxide as the sole carbon source. The other bacterium is a novel member of the family Spirochaetaceae. Characterization and manuscript preparation are currently underway. From SURF, we enriched a novel member of the phylum Thaumarchaeota; isolation attempts are underway, and lipid characterization will be conducted in collaboration with Roger Summons and the MIT CAN-6 team. Metagenome analysis from the same fluids has revealed two separate lineages of Thaumarchaeota. These genomes are currently being compared to genomes of marine and terrestrial Thaumarchaeota. Using the DHS technique, a novel sulfate reducing bacteria (SRB) has been isolated from SURF. The 16S gene sequence of the novel SRB was compared to 454 tag sequencing data from fluids at SURF and it was found that the cultured SRB is relatively dominant in situ, comprising ~5% of the total community. Metagenomic analysis confirms the prevalence of sulfate reduction as a major metabolic pathway employed in this deep subsurface location.
Electrode Cultivation (ex situ). Subsurface environments are geologically diverse, potentially allowing energy harvesting by microorganisms that catalyze redox reactions, but many of the abundant electron donors and acceptors are insoluble and therefore difficult to acquire energy from. Extracellular electron transfer is a metabolic strategy that microorganisms can deploy to meet the challenge of interacting with such redox-active surfaces. Since this process can be mimicked on electrode surfaces, it opens the door to electrochemical techniques to enrich for and quantify the activities of the relevant microorganisms. During years 1 and 2 of the project, graduate student Yamini Jangir in El-Naggar lab developed and applied an electrochemical platform to enrich for metal-reducing bacteria from a deeply-sourced artesian well in Death Valley, California (Nevares Deep Well 2). Within a chemically defined medium designed to mimic the natural site, four working electrodes were poised at different redox potentials (272, 373, 472, 572 mV vs. SHE) to serve as electron acceptors, resulting in anodic currents coupled to the oxidation of acetate during enrichment. The anodes were dominated by the Comamonadaceae and Rhodocyclaceae families from the Betaproteobacteria. During this reporting period (year 3), a representative of each dominant family was isolated from the electrode-associated biomass. The extracellular electron transfer abilities of the isolated Delftia strain (designated WE1) and Azonexus strain (designated WE2) were confirmed in electrochemical reactors using working electrodes poised at 522 mV vs. SHE. The rise in anodic current upon inoculation was correlated with a modest increase in total protein content from the cells. Both genera were previously observed in mixed communities of microbial fuel cell enrichments, but this is the first direct measurement of their electrochemical activity. While alternate metabolisms (e.g. nitrate reduction) by these organisms were previously known, our observations suggest that additional ‘hidden’ interactions with external electron acceptors are also possible. Electrochemical approaches are well positioned to dissect such extracellular interactions that may be prevalent in the subsurface (manuscript in preparation for Frontiers in Microbiology special issue – “Studies on life at the energetic edge”)
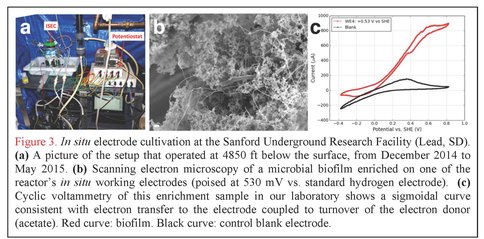
In situ Electrode Cultivation at Sanford Underground Research Facility. In addition to our laboratory-based efforts, graduate student Yamini Jangir (El-Naggar lab) has designed, constructed, and deployed the first experimental system of its kind: an in situ electrode cultivation (ISEC) bioreactor at 4850 ft below the surface, fed with water from legacy hole 3A at SURF in South Dakota. The system was operational from December 2014 to May 2015, and enriched for microbes at four redox conditions (-190, 10, 260, 530 mV vs. SHE simultaneously in one reactor) to mimic specific metabolisms, ranging from elemental sulfur oxidation to iron and manganese reduction, as predicted by our team’s recent modeling effort (Osburn et al., 2014). After decommissioning of ISEC, 454 pyrosequencing of the 16S rRNA transcripts was performed to assess the metabolically active microbial community from each electrode. By using an RNA-based approach targeting the active communities (rather than the overall taxonomical composition typically obtained using 16S rRNA gene sequencing, including dead and dormant cells), we will gain unprecedented insight into the diversity and prevalence of electrochemically active subsurface microbes (analysis currently in progress). Furthermore, these in situ electrodes were used as inocula for further laboratory-based (ex situ) electrochemical enrichments and mechanistic voltammetry studies (Figure 3); the metabolically active microbes from these samples are also being assessed using the same RNA-based approaches. In addition to these ongoing analyses (16S rRNA transcripts and cyclic voltammetry), we are currently isolating representative strains from each of these conditions.
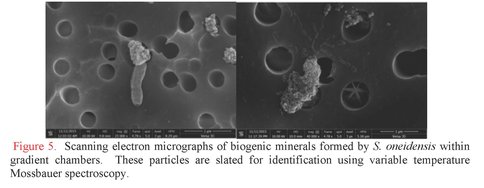
Components and mechanisms of extracellular charge transfer in subsurface microbes. Graduate student Lucas Lis at RPI has developed electrocultivation capabilities to support a wide range of subsurface metabolisms, including for dissimilatory iron-reducing and iron-oxidizing bacteria operating at pH values from 2.5 to 12. Lis constructed and instrumented gradient chambers with electrodes that can be independently poised to precise potential to serve either as anode or cathode to support bioenergetics (Figure 4). With this system, Lis investigated the production of biogenic minerals using the dissimilatory iron-reducing bacterium, Shewanella oneidensis as the catalytic agent. Lactate serves as the electron donor and diffuses upward into the chamber from a plug of 5% agarose that is solidified at the bottom. Poorly crystalline hydrous ferric oxide (HFO) was mixed with bacterial cells and the mixture was added to the chamber. Cells used for these inoculations were cultivated in chemostat cultures under conditions that promote the production of electrically-conductive filaments called bacterial nanowires. These structures help transmit electrons from cells to solid phase electron acceptors, like HFO and electrode surfaces. The chamber was then incubated in a hydrated environment under aerobic conditions to allow oxygen to dissolve in from the top. A spectral gradient of biogenic minerals is easily visible. Samples collected along this gradient were submitted to critical point drying and imaged by scanning electron microcopy, revealing a variety of biogenic minerals with sizes ranging from a few nanometers to several microns (Figure 5). Minerals will soon be analyzed and identified using variable temperature Mossbauer spectroscopy, which is available for use at the Environmental Molecular Science Laboratory in Richland, WA.
Gradient chambers will now be used to investigate metabolic activity, extracellular charge transfer, and mineralogical transformation by microbes recently isolated from the subsurface. A postdoctoral associate in the Moser lab, Scott Hamilton-Brehm, with partial support from this NAI node, isolated a number of organisms from the BLM-1site, including Desulfotomaculum putei (a sulfate-reducing Firmicute) and two novel Firmicutes that grow by fumarate fermentation and autotrophic sulfate reduction. Methanobacterium thermoautotrophicum (an autotrophic methanogen) represents another isolate that was previously shown to produce electrically conductive extracellular filaments when grown in co-culture with a thermophilic sulfate reducer. These organisms are perfect candidates for examining extracellular charge transfer under low energy conditions in gradient chambers.
D. Energy Flow and Metabolic Modeling.
Several projects in the energy flow and metabolic modeling theme made significant advances in 2015, resulting in six peer-reviewed articles (4 published, 2 in press) and inclusion in numerous oral presentations and university lectures. These projects include a) a study on the quantitative relationship between rates of microbial catalysis, energy supply and demand, and population size; b) the power limits of microbial life; c) a new view of extremophiles that specifically considers energetics; d) energy calculations of chemolithotrophic reactions in a hydrothermal system; e) the energetics of anabolism across a range of physico-chemical conditions; and f) a modeling effort of microbial limitations going back to the time of life’s emergence on Earth more than 3.5 billion years ago.
In LaRowe and Amend (2015a), we determined rates of biomass change as a function of the proportion of catabolic power that is converted into anabolism and the amount of energy that is required to synthesize biomass. Catabolic power is related to biomass through an energy-based yield coefficient that takes into account the constraints that different environments impose on biomolecule synthesis; this method is compared to other approaches for determining yield coefficients. Furthermore, so-called microbial maintenance energies that have been reported in the literature, which span many orders of magnitude, were reviewed. The equations developed in this study were used to demonstrate the interrelatedness of catabolic reaction rates, Gibbs energy of reaction, maintenance energy, biomass yield coefficients, microbial population sizes and doubling/replacement times.
In LaRowe and Amend (2015b), a bioenergetic model was applied to a well-characterized marine sedimentary environment in order to quantify the amount of power organisms use in an ultralow-energy setting. We showed a direct link between power consumption in this environment and the amount of biomass (cells/cm3) found in it. The power supply resulting from the aerobic degradation of particulate organic carbon (POC) at IODP site U1370 in the South Pacific Gyre is between ~ 10-12 and 10-16 W/cm3. The rates of POC degradation were calculated using a continuum model while Gibbs energies were computed using geochemical data describing the sediment as a function of depth.
In Marlow and Amend (2015), we note that if we can estimate how much energy is required for survival, and compare that to how much energy is available to be extracted from the environment, we can begin to consider “extreme” organisms in an objective fashion. Some of the most exotic environments actually offer luxurious energetic balances. It’s the microbes with low net energy availability that are the real extremophiles, whether they live an expensive existence in a high-energy environment, or an ascetic life in an energetic desert.
In Price et al. (2015), we calculated reaction energetics for chemolithotrophs in a hydrothermal system off Panarea Island (Italy) to determine how physical and chemical parameters influence the metabolic potential of the resident microbial communities. Gibbs energies (Δ_Gr_) of redox reactions that couple potential terminal electron acceptors (O2, NO3-, MnIV, FeIII, SO42-, S0, CO2,) with potential electron donors (H2, NH4+, Fe2+, Mn2+, H2S, CH4) were evaluated at in situ temperatures and compositions at several sites. When Gibbs energies of reaction are normalized per kilogram of hydrothermal fluid, sulfur oxidation reactions are the most exergonic, while the oxidation of Fe2+, NH4+, CH4, and Mn2+ are moderately energy yielding. The energetics calculations indicate that the most robust microbial communities in the Panarea hot springs combine H2S from deep water-rock-gas interactions with O2 that is entrained via seawater mixing to fuel their activities.
In LaRowe and Amend (2016), we quantified the amount of energy required to make biomass as a function of temperature, pressure, redox state, the sources of C, N and S, cell mass and the time that an organism requires to double or replace its biomass. Specifically, these energetics were calculated from 0 to 125 °C, 0.1 to 500 MPa and −0.38 to + 0.86 V using CO2, acetate or CH4 for C, NO3- or NH4+ for N and SO42-or HS- for S. The amounts of energy associated with synthesizing the biomolecules that make up a cell were then used to compute energy-based yield coefficients for a vast range of environmental conditions.
In Kempes et al. (2016), we presented a theory, which we verify using compiled data, for the changes in cellular composition across the entire range of bacteria cell sizes. Our analysis spans a huge diversity of species and 4 orders of magnitude in body size. We showed that the interconnection between energetic, physical, informational (genomic), chemical, and temporal limitations constrain the upper and lower boundaries of bacterial size and define the evolutionary flexibility for bacteria between these two bounds.
Publications
-
(no authors found) (2015). PERSPECTIVE. Elements, 11(6), 384–385. doi:10.2113/gselements.11.6.384
-
Case, D. H., Pasulka, A. L., Marlow, J. J., Grupe, B. M., Levin, L. A., & Orphan, V. J. (2015). Methane Seep Carbonates Host Distinct, Diverse, and Dynamic Microbial Assemblages. mBio, 6(6), e01348–15. doi:10.1128/mbio.01348-15
-
Dawson, K. S., Osburn, M. R., Sessions, A. L., & Orphan, V. J. (2015). Metabolic associations with archaea drive shifts in hydrogen isotope fractionation in sulfate-reducing bacterial lipids in cocultures and methane seeps. Geobiology, 13(5), 462–477. doi:10.1111/gbi.12140
-
Gross, B. J., & El-Naggar, M. Y. (2015). A combined electrochemical and optical trapping platform for measuring single cell respiration rates at electrode interfaces. Review of Scientific Instruments, 86(6), 064301. doi:10.1063/1.4922853
-
Inskeep, W. P., Jay, Z. J., MacUr, R. E., Clingenpeel, S., Tenney, A., Lovalvo, D., … Nealson, K. (2015). Geomicrobiology of sublacustrine thermal vents in Yellowstone Lake: geochemical controls on microbial community structure and function. Frontiers in Microbiology, 6. doi:10.3389/fmicb.2015.01044
-
Ishii, S. i., Suzuki, S., Tenney, A., Norden-Krichmar, T. M., Nealson, K. H., & Bretschger, O. (2015). Microbial metabolic networks in a complex electrogenic biofilm recovered from a stimulus-induced metatranscriptomics approach. Scientific Reports, 5, 14840. doi:10.1038/srep14840
-
LaRowe, D. E., & Amend, J. P. (2015). Catabolic rates, population sizes and doubling/replacement times of microorganisms in natural settings. American Journal of Science, 315(3), 167–203. doi:10.2475/03.2015.01
-
LaRowe, D. E., & Amend, J. P. (2015). Power limits for microbial life. Frontiers in Microbiology, 6. doi:10.3389/fmicb.2015.00718
-
Li, S-L., & Nealson, K. H. (2015). Enriching distinctive microbial communities from marine sediments via an electrochemical-sulfide-oxidizing process on carbon electrodes. Frontiers in Microbiology, 6. doi:10.3389/fmicb.2015.00111
-
Lin, H-T., Amend, J. P., LaRowe, D. E., Bingham, J-P., & Cowen, J. P. (2015). Dissolved amino acids in oceanic basaltic basement fluids. Geochimica et Cosmochimica Acta, 164, 175–190. doi:10.1016/j.gca.2015.04.044
-
Marlow, J., Peckmann, J., & Orphan, V. (2015). Autoendoliths: a distinct type of rock-hosted microbial life. Geobiology, 13(4), 303–307. doi:10.1111/gbi.12131
-
Mason, O. U., Case, D. H., Naehr, T. H., Lee, R. W., Thomas, R. B., Bailey, J. V., & Orphan, V. J. (2015). Comparison of Archaeal and Bacterial Diversity in Methane Seep Carbonate Nodules and Host Sediments, Eel River Basin and Hydrate Ridge, USA. Microbial Ecology, 70(3), 766–784. doi:10.1007/s00248-015-0615-6
-
McFarlane, I. R., Lazzari-Dean, J. R., & El-Naggar, M. Y. (2015). Field effect transistors based on semiconductive microbially synthesized chalcogenide nanofibers. Acta Biomaterialia, 13, 364–373. doi:10.1016/j.actbio.2014.11.005
-
McGlynn, S. E., Chadwick, G. L., Kempes, C. P., & Orphan, V. J. (2015). Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature, 526(7574), 531–535. doi:10.1038/nature15512
-
Nakano, C. M., Byun, H. S., Ma, H., Wei, T., & El-Naggar, M. Y. (2015). A framework for stochastic simulations and visualization of biological electron-transfer dynamics. Computer Physics Communications, 193, 1–9. doi:10.1016/j.cpc.2015.03.009
-
Nealson, K. H. (2015). Ex-phot: a new take on primitive utilization of solar energy. Environmental Microbiology Reports, 7(1), 33–35. doi:10.1111/1758-2229.12256
-
Osburn, M. R., Owens, J., Bergmann, K. D., Lyons, T. W., & Grotzinger, J. P. (2015). Dynamic changes in sulfate sulfur isotopes preceding the Ediacaran Shuram Excursion. Geochimica et Cosmochimica Acta, 170, 204–224. doi:10.1016/j.gca.2015.07.039
-
Price, R. E., LaRowe, D. E., Italiano, F., Savov, I., Pichler, T., & Amend, J. P. (2015). Subsurface hydrothermal processes and the bioenergetics of chemolithoautotrophy at the shallow-sea vents off Panarea Island (Italy). Chemical Geology, 407-408, 21–45. doi:10.1016/j.chemgeo.2015.04.011
-
Robador, A., Jungbluth, S. P., LaRowe, D. E., Bowers, R. M., Rappé, M. S., Amend, J. P., & Cowen, J. P. (2015). Activity and phylogenetic diversity of sulfate-reducing microorganisms in low-temperature subsurface fluids within the upper oceanic crust. Frontiers in Microbiology, 5. doi:10.3389/fmicb.2014.00748
-
Rowe, A. R., Chellamuthu, P., Lam, B., Okamoto, A., & Nealson, K. H. (2015). Marine sediments microbes capable of electrode oxidation as a surrogate for lithotrophic insoluble substrate metabolism. Frontiers in Microbiology, 5. doi:10.3389/fmicb.2014.00784
-
Skennerton, C. T., Ward, L. M., Michel, A., Metcalfe, K., Valiente, C., Mullin, S., … Orphan, V. J. (2015). Genomic Reconstruction of an Uncultured Hydrothermal Vent Gammaproteobacterial Methanotroph (Family Methylothermaceae) Indicates Multiple Adaptations to Oxygen Limitation. Frontiers in Microbiology, 6. doi:10.3389/fmicb.2015.01425
-
Stern, J. C., Sutter, B., Freissinet, C., Navarro-González, R., McKay, C. P., Archer, P. D., … Mahaffy, P. R. (2015). Evidence for indigenous nitrogen in sedimentary and aeolian deposits from the Curiosity rover investigations at Gale crater, Mars. Proc Natl Acad Sci USA, 112(14), 4245–4250. doi:10.1073/pnas.1420932112
-
Sylvan, J. B., Amend, J. P., Momper, L. M., Edwards, K. J., Toner, B. M., & Hoffman, C. L. (2015). Bacillus rigiliprofundi sp. nov., an endospore-forming, Mn-oxidizing, moderately halophilic bacterium isolated from deep subseafloor basaltic crust. International Journal of Systematic and Evolutionary Microbiology, 65(6), 1992–1998. doi:10.1099/ijs.0.000211
-
Thacher, R., Hsu, L., Ravindran, V., Nealson, K. H., & Pirbazari, M. (2015). Modeling the transport and bioreduction of hexavalent chromium in aquifers: Influence of natural organic matter. Chemical Engineering Science, 138, 552–565. doi:10.1016/j.ces.2015.08.011
-
White, L. M., Bhartia, R., Stucky, G. D., Kanik, I., & Russell, M. J. (2015). Mackinawite and greigite in ancient alkaline hydrothermal chimneys: Identifying potential key catalysts for emergent life. Earth and Planetary Science Letters, 430, 105–114. doi:10.1016/j.epsl.2015.08.013
-
Inagaki, F., Hinrichs, K-U., Kubo, Y., Bowles, M. W., Heuer, V. B., Hong, W-L., … Yamada, Y. (2015). Exploring deep microbial life in coal-bearing sediment down to 2.5 km below the ocean floor. Science, 349(6246), 420–424. doi:10.1126/science.aaa6882
-
Kieft, T. L., Onstott, T. C., Ahonen, L., Aloisi, V., Colwell, F. S., Engelen, B., … Wilkins, M. J. (2015). Workshop to develop deep-life continental scientific drilling projects. Sci. Dril., 19, 43–53. doi:10.5194/sd-19-43-2015
-
LaRowe, D. E., & Amend, J. P. (2016). The energetics of anabolism in natural settings. The ISME Journal, 10(6), 1285–1295. doi:10.1038/ismej.2015.227
-
McKay, L., Klokman, V. W., Mendlovitz, H. P., LaRowe, D. E., Hoer, D. R., Albert, D., … Teske, A. (2016). Thermal and geochemical influences on microbial biogeography in the hydrothermal sediments of Guaymas Basin, Gulf of California. Environmental Microbiology Reports, 8(1), 150–161. doi:10.1111/1758-2229.12365
-
Salas, E. C., Bhartia, R., Anderson, L., Hug, W. F., Reid, R. D., Iturrino, G., & Edwards, K. J. (2015). In situ Detection of Microbial Life in the Deep Biosphere in Igneous Ocean Crust. Frontiers in Microbiology, 6. doi:10.3389/fmicb.2015.01260
- Bhartia, R., W.H. Hug, R.Reid, L. Beegle. (2015). Explosives Detection and Analysis by Fusing Deep UV Native Fluorescence and Resonance Raman Spectroscopy. In E. L. H. P.M. Pellegrino, M.E. Ferrell (Ed.), Laser-based Optical Detection methods of Explosives. Boca Raton, FL: Taylor & Francis Group.
- Marlow, J., & Amend, J. (2015). The Energy of Life. Scientist, 29(2), 40-47.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Rohit Bhartia
Co-Investigator
Moh El-Naggar
Co-Investigator
Tracy Fullerton
Co-Investigator
Yuri Gorby
Co-Investigator
Duane Moser
Co-Investigator
Kenneth Nealson
Co-Investigator
Victoria Orphan
Co-Investigator
Magdalena Osburn
Co-Investigator
Holly Willis
Co-Investigator
William Abbey
Collaborator
Anja Bauermeister
Collaborator
Lina Bird
Collaborator
Sean Bouchard
Collaborator
David Case
Collaborator
Gray Chadwick
Collaborator
Stephanie Connon
Collaborator
Bethany Ehlmann
Collaborator
Bethany Ehlmann
Collaborator
Scott Hamilton-Brehm
Collaborator
Fumio Inagaki
Collaborator
Yamini Jangir
Collaborator
Chris Kempes
Collaborator
Brittany Kruger
Collaborator
J. Gijs Kuenen
Collaborator
Doug LaRowe
Collaborator
Bonita Lam
Collaborator
Lucas Lis
Collaborator
Jeffrey Marlow
Collaborator
Urbashi Mitra
Collaborator
Lily Momper
Collaborator
Penny Morrill
Collaborator
Sean Mullin
Collaborator
Akihira Okamoto
Collaborator
Beth Orcutt
Collaborator
Veronica Paez
Collaborator
Sahand Pirbadian
Collaborator
Brandi Reese
Collaborator
Nerissa Rivera
Collaborator
Alberto Robador
Collaborator
Tyler Roche
Collaborator
Karyn Rogers
Collaborator
Annette Rowe
Collaborator
Joshua Sackett
Collaborator
Pratixa Savalia
Collaborator
Shakher Sijapati
Collaborator
Shino Suzuki
Collaborator
Shino Suzuki
Collaborator
Elizabeth Swenson
Collaborator
Elizabeth Trembath-Reichert
Collaborator
Greg Wanger
Collaborator
Laura Zinke
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 4.1
Earth's early biosphere.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.2
Biosignatures to be sought in nearby planetary systems