2015 Annual Science Report
 Massachusetts Institute of Technology
Reporting | JAN 2015 – DEC 2015
Massachusetts Institute of Technology
Reporting | JAN 2015 – DEC 2015
Progress in the Elucidation of Microbial Biosignatures
Project Summary
A number of discrete individual investigations have contributed to improved knowledge about the occurrence and interpretation of microbial molecular biosignatures across all geological timescales.
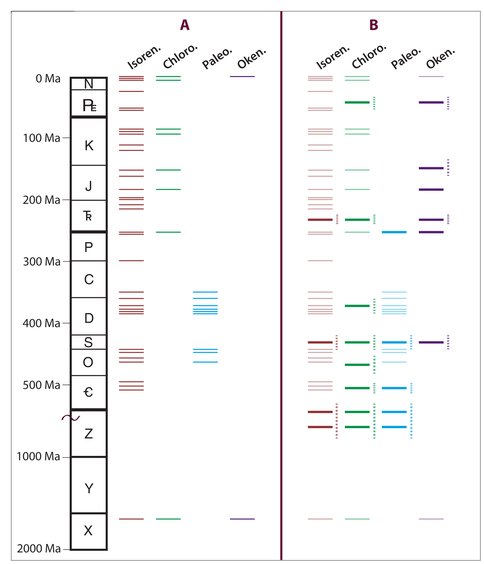
A new analytical approach enabled a revised geologic distributions of fossilized biomarkers for anoxygenic sulfur bacteria. The prevalence of okenane and chlorobactane suggests that marine photic zone euxinia (PZE) was more intense and frequent in the geologic past. However, the presence of these compounds in some sediments and oils may also be a signature for basin restriction rather than one indicating more widespread marine anoxia.
In a related work, pervasive photic zone euxinia and disruption of biogeochemical cycles was demonstrated for a sequence of rocks deposited on the northeastern Panthalassic Ocean during the end-Triassic extinction.
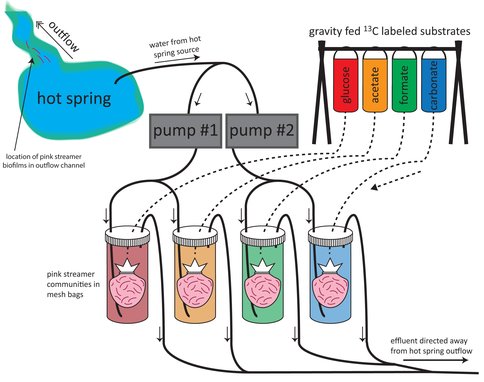
A study of lipids and their isotopic compositions, combined with stable isotope probing experiments, demonstrated that streamer biofilm communities, which are a present in the high temperature zones of hydrothermal features of the Lower Geyser Basin of Yelowstone National Park, can alternate their metabolism between autotrophy and heterotrophy depending on substrate availability.
Other collaborations with numerous colleagues resulted in documentation of lipid and isotopic biosignatures in cultured bacteria.
Project Progress
Marine rock extracts and oils from the Proterozoic to the Paleogene were analyzed for intact saturated and aromatic carotenoid carbon skeletons using new analytical methods. Gas chromatography coupled with tandem mass spectrometry revealed new temporal distribution patterns for chlorobactane, paleorenieratane, and okenane. One interpretation of the revised geologic distributions of these fossilized biomarkers for GSB and PSB, especially okenane and chlorobactane, is that marine photic zone euxinia (PZE) was more intense and frequent in the geologic past, particularly the Phanerozoic, than was previously thought. However, given that okenane and chlorobactane are commonly interpreted to imply hydrogen sulfide at water depths shallower than 24 m, their common occurrence in Phanerozoic marine samples presents several challenges given that atmospheric oxygen levels were near modern values during the time of deposition. Therefore, the prevalence of okenane and chlorobactane in ancient marine systems likely reflects the degree of basin restriction and not global oceanographic redox conditions (Figure 1).
Fossil molecular (biomarker) and stable nitrogen isotopic records in a sedimentary sequence from western Canada provide the first conclusive evidence of PZE and disrupted biogeochemistry on the northeastern Panthalassic Ocean for the end-Triassic. These results indicate the occurrence of increasing water column stratification and deoxygenation across the ETE, leading to PZE in the Early Jurassic. The development of PZE was paralleled by a perturbed nitrogen cycle, ecological turnovers among non-calcifying groups including eukaryotic algae and prokaryotic plankton.
We studied the lipid and C-isotopic composition of s treamer biofilm communities (SBC) within chemosynthetic zones of Yellowstone hot spring outflow channels, where temperatures exceed those conducive to photosynthesis. Nearest the hydrothermal source (75–88◦C) SBC comprise thermophilic Archaea and Bacteria, often mixed communities including Desulfurococcales and uncultured Crenarchaeota, as well as Aquificae, Thermus, each carrying diagnostic membrane lipid biomarkers. We tested the hypothesis that SBC can alternate their metabolism between autotrophy and heterotrophy depending on substrate availability. Feeding experiments were performed at two alkaline hot springs in Yellowstone National Park: Octopus Spring and “Bison Pool,” using various 13C-labeled substrates (bicarbonate, formate, acetate, and glucose) to determine the relative uptake of these different carbon sources. Highest 13C uptake, at both sites, was from acetate into almost all bacterial fatty acids, particularly into methyl-branched C15, C17 and C19 fatty acids that are diagnostic for Thermus/Meiothermus, and some Firmicutes as well as into universally common C16:0 and C18:0 fatty acids. 13C-glucose showed a similar, but a 10–30 times lower uptake across most fatty acids. 13C-bicarbonate uptake, signifying the presence of autotrophic communities was only significant at “Bison Pool” and was observed predominantly in non-specific saturated C16, C18, C20, and C22 fatty acids. Incorporation of 13C-formate occurred only at very low rates at “Bison Pool” and was almost undetectable at Octopus Spring, suggesting that formate is not an important carbon source for SBC. 13C-uptake into archaeal lipids occurred predominantly with 13C-acetate, suggesting also that archaeal communities at both springs have primarily heterotrophic carbon assimilation pathways. We hypothesize that these communities are energy-limited and predominantly nurtured by input of exogenous organic material, with only a small fraction being sustained by autotrophic growth.
Other collaborations with numerous colleagues resulted in documentation of lipid and isotopic biosignatures in cultured methanotrophic and planctomycete bacteria.
Lastly, we studied the lipid and isotopic compositions of the microbial communities colonizing ooids and developed a tool to measure, at high resolution, chronologies of their growth using radiocarbon analysis.
Publications
-
Baesman, S., Miller, L., Wei, J., Cho, Y., Matys, E., Summons, R., … Oremland, R. (2015). Methane Oxidation and Molecular Characterization of Methanotrophs from a Former Mercury Mine Impoundment. Microorganisms, 3(2), 290–309. doi:10.3390/microorganisms3020290
-
Beaupré, S. R., Roberts, M. L., Burton, J. R., & Summons, R. E. (2015). Rapid, high-resolution 14C chronology of ooids. Geochimica et Cosmochimica Acta, 159, 126–138. doi:10.1016/j.gca.2015.03.009
-
Bender, M., Schmidtmann, M., Summons, R. E., Rullkötter, J., & Christoffers, J. (2015). A Geomimetic Approach to the Formation and Identification of Fossil Sterane Biomarkers in Crude Oil: 18- nor – D – homo -Androstane and 5α,14β-Androstane. Chem. Eur. J., 21(35), 12501–12508. doi:10.1002/chem.201502148
-
Escobedo-Hinojosa, W. I., Vences-Guzmán, M. Á., Schubotz, F., Sandoval-Calderón, M., Summons, R. E., López-Lara, I. M., … Sohlenkamp, C. (2015). OlsG (Sinac_1600) Is an Ornithine Lipid N -Methyltransferase from the Planctomycete Singulisphaera acidiphila. J. Biol. Chem., 290(24), 15102–15111. doi:10.1074/jbc.m115.639575
-
French, K. L., Rocher, D., Zumberge, J. E., & Summons, R. E. (2015). Assessing the distribution of sedimentary C 40 carotenoids through time. Geobiology, 13(2), 139–151. doi:10.1111/gbi.12126
-
Kasprak, A. H., Sepulveda, J., Price-Waldman, R., Williford, K. H., Schoepfer, S. D., Haggart, J. W., … Whiteside, J. H. (2015). Episodic photic zone euxinia in the northeastern Panthalassic Ocean during the end-Triassic extinction. Geology, 43(4), 307–310. doi:10.1130/g36371.1
-
Schubotz, F., Hays, L. E., Meyer-Dombard, D. A. R., Gillespie, A., Shock, E. L., & Summons, R. E. (2015). Stable isotope labeling confirms mixotrophic nature of streamer biofilm communities at alkaline hot springs. Frontiers in Microbiology, 6. doi:10.3389/fmicb.2015.00042
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Katherine French
Co-Investigator
Florence Schubotz
Co-Investigator
Julio Sepulveda
Co-Investigator
Paula Welander
Co-Investigator
Jessica Whiteside
Co-Investigator
Steven Beaupre
Collaborator
Joshua Burton
Collaborator
Lindsay Hays
Collaborator
Alex Kasprak
Collaborator
Emily Matys
Collaborator
D'Arcy Meyer-Dombard
Collaborator
Mark Roberts
Collaborator
Don Rocher
Collaborator
Everett Shock
Collaborator
Kenneth Williford
Collaborator
John Zumberge
Collaborator
-
RELATED OBJECTIVES:
Objective 3.2
Origins and evolution of functional biomolecules
Objective 4.2
Production of complex life.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems