2015 Annual Science Report
 NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | JAN 2015 – DEC 2015
NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | JAN 2015 – DEC 2015
Inv 4 - Observable Chemical Signatures on Icy Worlds: A Window Into Habitability of Subsurface Oceans
Project Summary
INV 4 aims to answer the Key Question: What can observable surface chemical signatures tell us about the habitability of subsurface oceans? We will shed light on the evolution of ocean materials expressed on the surface of airless icy bodies and exposed to relevant surface temperatures, vacuum, photolysis and radiolysis. To this end, we have initiated an experimental program designed to establish the extent to which chemical compositions of icy world surfaces are indicative of subsurface ocean chemistry. Our initial experiments have focused on freezing solutions of sodium, magnesium, sulfate and chloride – four commonly suggested major components of Europa’s Ocean.
Project Progress
The composition of Europa’s subsurface ocean is a critical determinant of its habitability. However, our current understanding of the ocean composition is limited to its expression on the surface. We have begun to experimentally investigate the composition of mixed sodium–magnesium–sulfate–chloride solutions when frozen to 100 K, simulating conditions that likely occur as ocean fluids are emplaced onto Europa’s surface. Our goal is to systematically examine how chemical composition of Europa’s surface reflects subsurface ocean chemistry and composition, and thus enable constraints to be placed on ocean composition through the observation of surface materials.
Frozen Na+-Mg2+-Cl--SO42- brines were investigated using a confocal dispersive Raman spectrometer (Horiba Jobin-Yvon LabRam HR) with cryogenic optical stage (Linkam LTS 350). Ten microliter droplets of solution were placed inside the cryostage on a glass microscope slide, and cooled to the temperature of interest. In some cases, samples were then annealed at a higher temperature before recooling.
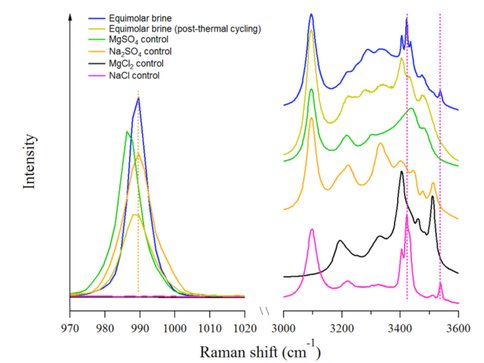
Figure 1 shows the results of experiments examining the freezing behavior of a solution composed of a 1:1 mixture of saturated solutions of NaCl and MgSO4. The molar concentrations of the ions in this solution are given in Table 1 (sat. experiment). The onset of freezing, indicated by the appearance of crystals, occurred at 211 K.
Figure 1 compares the spectrum of the sat. solution with the spectra of control solutions composed of saturated solutions of MgSO4, Na2SO4, NaCl, and MgCl2. We identify the peak at 990 cm-1 as the υ1 symmetric stretching mode of the sulfate anion. This position is diagnostic for the formation of mirabilite, Na2SO4•10H2O [2]. Other features of interest include the sharp peaks around 100–200 cm−1 and especially at ∼3420 cm−1. These likely indicate presence of both MgCl2 (black dotted line) and NaCl (magenta dotted line). The feature at 3420 cm−1 is indicative of hydrohalite (NaCl•2H2O) [3]. MgCl2 is likely also hydrated.
The sat. solution discussed above contains an excess of Na+, perhaps biasing the results towards the formation of mirabilite. To test this possibility, we performed an experiment with 1M each of Na+, Mg2+, Cl− and SO4-2 frozen to 100 K. In this solution, stoichiometry of the salts would yield an excess of Mg2+ and SO4-2 in the solution. The results are shown in Figure 2.
Again, the frequency of the υ1 sulfate stretch at 990 cm-1 indicates the formation of mirabilite. Peaks at 3420 and 3536 cm−1 (magenta dotted lines) point to the formation of hydrohalite. The sample was subsequently warmed to its melting temperature (263 K) allowing the sample to equilibratebefore refreezing it at 100 K. After thermal cycling virtually all of the remaining hydrohalite had melted and did not recrystallize upon subsequent cooling, suggesting that this species is not to be expected at thermodynamic equilibrium.
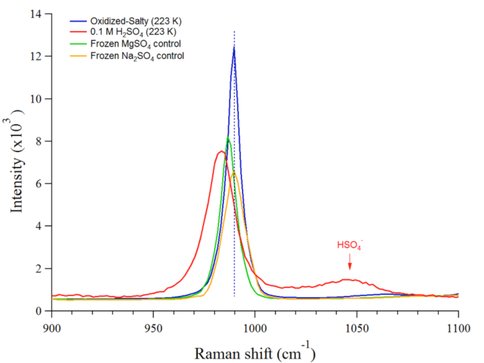
Figure 3 shows the results of an experiment using the “oxidized-salty” composition defined in Table 1. This solution more closely mimics the possible composition if the Europan ocean. The same behavior is observed with this solution as with the sat. solution and the equimolar solution. This solution was highly acidic (pH ~1), so a 0.1 M H2SO4 solution was frozen to 233 K for comparison. The υ1 sulfate stretch peak is again found at 990 cm-1, indicating the formation of mirabilite.
Our results indicate that solutions containing Na+, Mg2+, Cl-, SO42- form Na2SO4 and MgCl2 preferentially on freezing. Epsomite (MgSO4.7H2O) cannot form directly upon freezing of an Na-rich ocean unless the SO42- concentration is more than half that of Na+. Alternatively, Radiolytic processing, with implantation of sulfur ions originating from Io’ s volcanic eruptions, then transforms MgCl2 into epsomite as suggested by Brown & Hand (2013) [4].
If NaCl is present on the surface, as suggested by Hand & Carlson (2015) or Na+, Mg2+, Cl-, and SO42- concentrations are comparable, and the freezing rate is rapid.
This work and its implications are discussed in a publication in press at the time this report was written [6]. Future work will examine the effects of vacuum and radiolytic processing.
References:
[1]Marion, G. M., et al. (2005) GeCoA, 69, 259.
[2] Hamilton, A., and Menzies, R. I. (2010) JRSp, 41, 1014.
[3] Samson, I. M., and Walker, R. T. (2000) Can. Mineral., 38, 35.
[4] Brown, M. E., & Hand, K. P. (2013) AJ, 145, 110.
[5] Hand, K. P., and Carlson, R. W. (2015) GeoRL, 42, 3174.
[6] Vu, T.H., et al (2016). ApJLett, 816:L26 (6pp).
Publications
-
Vu, T. H., Hodyss, R., Choukroun, M., & Johnson, P. V. (2016). CHEMISTRY OF FROZEN SODIUM–MAGNESIUM–SULFATE–CHLORIDE BRINES: IMPLICATIONS FOR SURFACE EXPRESSION OF EUROPA’S OCEAN COMPOSITION. The Astrophysical Journal, 816(2), L26. doi:10.3847/2041-8205/816/2/l26
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Mathieu Choukroun
Co-Investigator
Robert Hodyss
Co-Investigator
Tuan Vu
Co-Investigator
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 7.1
Biosignatures to be sought in Solar System materials
![Table 1. Concentrations in molar (M) for various ions in the experimental mixtures. Sat.: a 1:1 mixture of saturated NaCl and MgSO4. Eq. M: an equimolar mixture of Na+, Mg2+ Cl- and SO42-. Ox-salt: the “oxidized-salty” composition from [1]](../../../../media/medialibrary/2016/03/Table1.jpg.484x0_q85.jpg)
![Figure 1: Comparison of the Raman spectrum of a frozen brine mixture containing 3.07 M [Na+], 3.07 M [Cl-], 1.44 M [Mg2+] and 1.44 M [SO42-] (blue) to that of frozen MgSO4 (green), Na2SO4 (orange), MgCl2 (black), and NaCl (magenta) control solutions at 211 K. Inset shows magnification of the sulfate stretch, illustrating the presence of Na2SO4 in the frozen brine.](../../../../media/medialibrary/2016/03/Figure1_0ZKEWts.jpg.484x0_q85.jpg)