2015 Annual Science Report
 NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | JAN 2015 – DEC 2015
NASA Jet Propulsion Laboratory - Icy Worlds
Reporting | JAN 2015 – DEC 2015
Inv 1 - Geochemical Reactor: Energy Production at Water-Rock Interfaces
Project Summary
INV 1 examines water-rock interactions in the lab and in the field, to characterize the geochemical gradients that could be present at water-rock interfaces on Earth and other worlds, taking into account different ocean and crustal chemistries. We have fully investigated serpentinization as the most likely of all possible environments for life’s emergence on Earth as well as other water-rich worlds – a key goal for astrobiology as stated in the NASA Astrobiology Roadmap 2008. (Russell, 2015). Serpentinization is now recognized as fundamental to delivering the appropriate chemical disequilibria at the emergence of life. And the fact that this process is likely inevitable on any icy, wet and rocky planet makes its study fundamental to emergence of life, habitability and habitancy. Nevertheless, notwithstanding the thermodynamic drives to CO2 reduction during the process, great uncertainty exists over just what kind of organic molecules (if any) are delivered to the submarine springs and consequential precipitate mounds. In attempts to clarify what these might be we have undertaken thermodynamic modelling and experimental investigations of the serpentinization process.
Project Progress
Our earlier findings drove us to look at the assumption that emergent life merely took over and quickened serpentinization reactions that prefigured the first metabolic pathways (Shibuya et al., 2016; White et al., 2016 in prep). It became clear that INV 2, with its emphasis on the transition from geochemistry to biochemistry and electrochemical experiments, should provide more of the answers (Barge et al., 2015; Burcar et al., 2015). On our part, we had to work out how biochemistry differed from the mass action chemistry normally assumed to account for early metabolism, a view that now looked inadequate in the light of our experiments. To this end, and in collaboration with Elbert Branscomb, Nigel Goldenfeld and Tommaso Biancanali of the NAI Universal Biology at University of Illinois Urbana Champaign, we investigated the mechanism of the simplest but vital enzymes at the gateways to primeval metabolic pathways and cycles (Branscomb et al., in review). Often presumed to be merely complex catalysts, it turns out that all enzymes are actually engines which couple endergonic (up hill reactions) with necessarily larger exergonic (downhill reactions) ones. As one fundamental and supposedly simple example – hydrogenase – is a “flippamer” complex with many moving parts (Ogata et al. 2015). Our research to date then has led to a reconceptualization of the issues facing experimental approaches to the emergence of life grounded in the idea that we are necessarily looking for mineral clusters able to change conformation in order to receive and/or deliver electrons (as in flippamers) or act as peristaltic pumps to facilitate reductions and oxidations (as in the flippases, see https://www.youtube.com/watch?v=pMoWPyKmKLY).
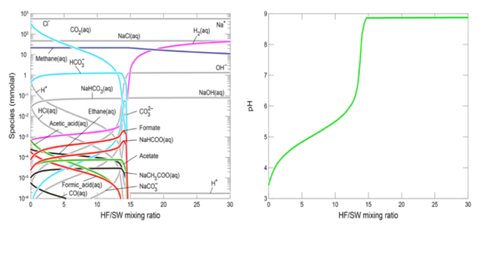
Thermodynamic expectations for the planned serpentinization experiments, with especial emphasis on methane, were calculated using the REACT module in Geochemist’s Workbench with a default thermodynamic database (Shibuya et al., 2016). The seawater solutions were equilibrated by ~10 and ~1 bar of CO2 gas just before the experiments 2 and 3, which correspond to approximately 340 and 34 mmol/kg of total CO2 (= CO2(aq) + HCO3− + CO32− + NaHCO3(aq) + NaCO3−) concentrations, respectively. From this thermodynamic modeling of mixing reactions at 120 °C, we can predict maximum methane concentrations of 22 mmol/kg in the regions of low HF/SW mixing ratio for both experiments (Figure 1).
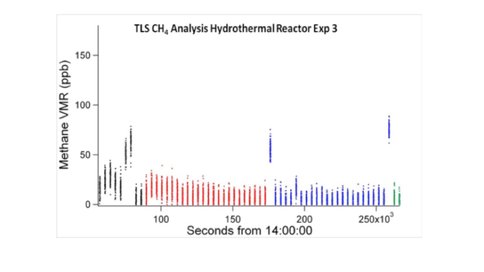
Furthermore, the results show that acetic acid species (e.g, acetic acid, acetate and NaCH3COOaq) and formic acid species (formic acid, formate and NaHCOO) can be also formed in the regions of low HF/SW mixing ratio although their concentrations are generally less than 1 μmol/kg. In experiments undertaken in the same conditions we demonstrated the reduction/hydrogenation of carbon dioxide to formate in a matter of minutes in hydrothermal serpentinization experiments. Nevertheless, using the same tunable diode laser spectrometer operated by MSL and involving radio-labelled carbon dioxide, methane could NOT be generated in over 100 hours of hydrothermal operation (White et al., 2016 in prep.).
These results contradict our earlier expectations that such a reduction to CH4 would be facile and quick. In the light of these findings we now assert that serpentinization is not the living fossil ‘sister group’ in the form of geochemical methane synthesis at modern hydrothermal vents. Rather, serpentinization can be considered the ‘mother engine’ that spawned life’s emergence. Under this new view, methane was not the first waste product of a protmethanogenic reaction how the first pathway to carbon fixation was channeled and negotiated, but, along with hydrogen, was the first fuel in what wwe term “proto-denitrfying metanotrophic acetogenesis”. Therefore, as a test of this alternative methanotrophic pathway we will, in conjunction with Investigation 2, attempt to oxidize hydrothermal methane to a methyl group with nitrate, involving green rust, greigite and mackinawite (White et al, 2015).
To further understand how such disequilibria might be converted and drive this type of metabolism we are collaborating with the NAI CAN6 University of Illinois Team “Towards Universal Biology” (Branscomb et al., 2016 in review; Branscomb & Russell, 2016).
References:
Branscomb, E. & Russell, M.J. (2016). Fluctuation relations, Bioenergetics and the emergence of life: The engines of creation. Gordon Research Conference, Galveston 17th – 22nd January 2016.
Branscomb, E. Biancalani, T. Goldenfeld N. & Russell M.J. Escapement Mechanisms and the Conversion of Disequilibria the engines of creation. To be submitted.
Ogata et al. (2015). Hydride bridge in [NiFe]-hydrogenase observed by nuclear resonance vibrational spectroscopy. Nature communications, 6, 7890.
Shibuya, T., Russell, M. J., & Takai, K. (2016). Free energy distribution and hydrothermal mineral precipitation in Hadean submarine alkaline vent systems: Importance of iron redox reactions under anoxic conditions. Geochimica et Cosmochimica Acta, 175, 1-19.
White et al. (2016). Methane: Fuel or exhaust at the emergence of life? in prep. for Astrobiology.
Publications
-
Barge, L. M., Abedian, Y., Russell, M. J., Doloboff, I. J., Cartwright, J. H. E., Kidd, R. D., & Kanik, I. (2015). From Chemical Gardens to Fuel Cells: Generation of Electrical Potential and Current Across Self-Assembling Iron Mineral Membranes. Angew. Chem. Int. Ed., 54(28), 8184–8187. doi:10.1002/anie.201501663
-
Branscomb, E., Biancalani, T., Goldenfeld, N., & Russell, M. (2017). Escapement mechanisms and the conversion of disequilibria; the engines of creation. Physics Reports. doi:10.1016/j.physrep.2017.02.001
-
Burcar, B. T., Barge, L. M., Trail, D., Watson, E. B., Russell, M. J., & McGown, L. B. (2015). RNA Oligomerization in Laboratory Analogues of Alkaline Hydrothermal Vent Systems. Astrobiology, 15(7), 509–522. doi:10.1089/ast.2014.1280
-
Russell, M. (2015). EMERGENCE OF LIFE AND ITS EARLY HISTORY. Ehrlich’s Geomicrobiology, Sixth Edition, None, 19–54. doi:10.1201/b19121-4
-
Shibuya, T., Russell, M. J., & Takai, K. (2016). Free energy distribution and hydrothermal mineral precipitation in Hadean submarine alkaline vent systems: Importance of iron redox reactions under anoxic conditions. Geochimica et Cosmochimica Acta, 175, 1–19. doi:10.1016/j.gca.2015.11.021
-
White, L. M., Bhartia, R., Stucky, G. D., Kanik, I., & Russell, M. J. (2015). Mackinawite and greigite in ancient alkaline hydrothermal chimneys: Identifying potential key catalysts for emergent life. Earth and Planetary Science Letters, 430, 105–114. doi:10.1016/j.epsl.2015.08.013
-
Duval, S., Santini, J. M., Lemaire, D., Chaspoul, F., Russell, M. J., Grimaldi, S., … Schoepp-Cothenet, B. (2016). The H-bond network surrounding the pyranopterins modulates redox cooperativity in the molybdenum-bisPGD cofactor in arsenite oxidase. Biochimica et Biophysica Acta (BBA) – Bioenergetics, 1857(9), 1353–1362. doi:10.1016/j.bbabio.2016.05.003
- White, L.M., Shibuya, T., Willis, P.A., Stockton, A.M., Cable, M.E., Christensen, L.E., Bhartia, R., Kidd, R., Vance, S., Mielke, R.E., Hoffman, A., Kanik, I., Russell M.J. Methane: Fuel or exhaust at the emergence of life? (To be submitted to Astrobiology)
- Wong, M.L., Charany, B.D., Gao, P., Yung, Y.L., Russell, M.J., Nitrogen oxides in early Earth’s atmosphere as electron acceptors for life’s emergence (in review Astrobiology).
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Lance Christensen
Co-Investigator
Steven Vance
Co-Investigator
Peter Willis
Co-Investigator
Takazo Shibuya
Collaborator
-
RELATED OBJECTIVES:
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules
Objective 3.3
Origins of energy transduction
Objective 3.4
Origins of cellularity and protobiological systems
Objective 4.1
Earth's early biosphere.