2015 Annual Science Report
 NASA Goddard Space Flight Center
Reporting | JAN 2015 – DEC 2015
NASA Goddard Space Flight Center
Reporting | JAN 2015 – DEC 2015
Laboratory Investigations Into Chemical Evolution in Icy Solids: Mars, Carbonaceous Meteorites, and ISM
Project Summary
The goal of this project is to investigate chemical and physical changes and properties of molecules in low-temperature environments, such as found in interstellar space and the outer regions of the Solar System. Some of the molecules studied have been detected in meteorites and samples returned from NASA missions.
4
Institutions
3
Teams
6
Publications
0
Field Sites
Project Progress
- NAI-GCA support in 2015 helped us continue our work on amino-acid stability. In 2015, we completed our first set of radiation experiments to measure the destruction rate of glycine in CO2 ice. Our results showed that glycine at a subsurface depth of 5-10 meters embedded in CO2-ice on Mars would not survive more than 100 million years, but could survive for 2-4 Gyr if stored in H2O-ice. This work continued activities from 2014 and was recently published in Icarus (Gerakines, P. A., & Hudson, R. L. (2015). The radiation stability of glycine in solid CO2 – In situ laboratory measurements with applications to Mars. Icarus, 252, 466–472. doi:10.1016/j.icarus.2015.02.008).
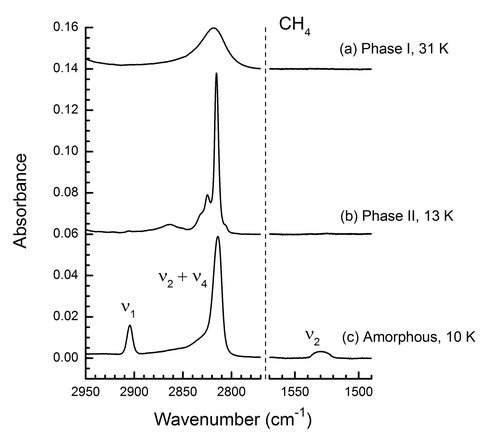
- We built on last year’s study of IR band strengths of ices made of organic molecules, as part of our interest in the carbon inventory of the outer Solar System and the interstellar medium. To extend this work we examined CH4 the simplest hydrocarbon, looking at each of its three solid phases. We discovered that all of the previous low-temperature publications on solid CH4 were for the compound’s crystalline phases and not the amorphous one that was usually claimed and which is more astronomically relevant. Reassessments of results from many earlier laboratory and observational papers are needed in light of our new work. Figure 1 shows spectral markers that are now known to distinguish amorphous and crystalline CH4 (Gerakines, P. A., & Hudson, R. L. (2015). Infrared spectra and optical constants of elusive amorphous methane. The Astrophysical Journal, 805(2), L20. doi:10.1088/2041-8205/805/2/l20).
Figure 1: Spectral features used to demonstrate the amorphous and crystalline nature of CH4 ices. The phases of greatest astrobiological and astrochemical interest are the amorphous and phase II forms. The ν1 and ν2 peaks are forbidden transitions that serve as spectral markers for amorphous CH4 (Gerakines and Hudson 2015).
- Building on our CH4 study, we turned to the other molecular extreme of carbon oxidation, CO2, finding that the situation regarding IR intensities and spectra was even worse than with CH4. Again, what had been assumed by astrochemists for about 30 years to be IR spectra of amorphous CO2 was actually for the crystalline solid. We succeeded in producing the amorphous material and publishing the results, the first such work available for CO2 (Gerakines, P. A., & Hudson, R. L. (2015). First infrared band strengths for amorphous CO2, an overlooked component of interstellar ices. The Astrophysical Journal, 808(2), L40. doi:10.1088/2041-8205/808/2/l40).
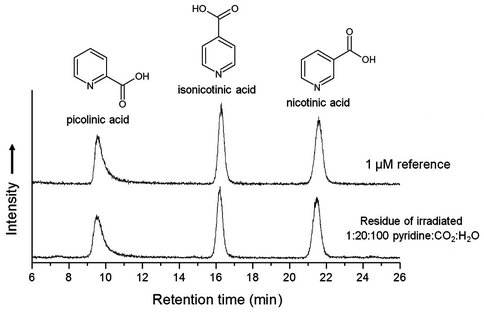
- In collaboration with scientists in NASA Goddard’s Astrobiology Analytical Laboratory we completed a new project concerning heterocycle chemistry in meteorites. Our first molecular targets were nicotinic acid and its isomers. An initial paper was published in 2014, followed by a more-detailed one in this reporting year (Smith, K. E., Gerakines, P. A., & Callahan, M. P. (2015). Metabolic precursors in astrophysical ice analogs: implications for meteorites and comets. Chem. Commun., 51(59), 11787–11790. doi:10.1039/c5cc03272e). In that paper we demonstrated that cosmic radiation acting on ices containing CO2 and pyridine, two known meteoritic molecules, would produce quinolinic and nicotinic acids, molecules that participate in the nicotinamide adenine dinucleotide (NAD) biosynthetic pathway. Figure 2 shows chromatograms of radiation-produced nicotinic acid (vitamin D) and two of its heterocyclic isomers.
Figure 2: Comparison of ion chromatograms at (m/z) = 124.0393 (5 ppm window) of reference standards to the organic residue formed by 1-MeV proton irradiation of a H2O + CO2 + pyridine (100:20:1) ice mixture. The incident radiation dose for both samples was 1 × 1015 p+ cm−2 (Smith, Gerakines, and Callahan 2015).
Publications
- Gerakines, P. A., & Hudson, R. L. (2015). First infrared band strengths for amorphous CO2, an overlooked component of interstellar ices. The Astrophysical Journal, 808(2), L40. doi:10.1088/2041-8205/808/2/l40
- Gerakines, P. A., & Hudson, R. L. (2015). Infrared spectra and optical constants of elusive amorphous methane. The Astrophysical Journal, 805(2), L20. doi:10.1088/2041-8205/805/2/l20
- Gerakines, P. A., & Hudson, R. L. (2015). The radiation stability of glycine in solid CO2 – In situ laboratory measurements with applications to Mars. Icarus, 252, 466–472. doi:10.1016/j.icarus.2015.02.008
- Hudson, R. L., Gerakines, P. A., & Loeffler, M. J. (2015). Activation of weak IR fundamentals of two species of astrochemical interest in the Td Point Group – the importance of amorphous ices. Phys. Chem. Chem. Phys., 17(19), 12545–12552. doi:10.1039/c5cp00975h
- Loeffler, M. J., & Hudson, R. L. (2015). Descent without modification? The thermal chemistry of H2O2 on Europa and other Icy Worlds. Astrobiology, 15(6), 453–461. doi:10.1089/ast.2014.1195
- Smith, K. E., Gerakines, P. A., & Callahan, M. P. (2015). Metabolic precursors in astrophysical ice analogs: implications for meteorites and comets. Chem. Commun., 51(59), 11787–11790. doi:10.1039/c5cc03272e
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Perry Gerakines
Co-Investigator
Mark Loeffler
Collaborator
Karen Smith
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts