2015 Annual Science Report
 University of Colorado, Boulder
Reporting | JAN 2015 – DEC 2015
University of Colorado, Boulder
Reporting | JAN 2015 – DEC 2015
Variable Generation of H2, Formate, and Critical Electron-Donors During Water/rock Reaction
Project Summary
The chemosynthetic microbial communities that inhabit peridotite-hosted springs and hydrothermal systems are supported by molecular hydrogen (H2), formate, and other electron donors that are generated by subsurface fluid-rock interactions during serpentinization. The objective of this project is to understand the sources, abundances, and diversity of critical electron donors produced during serpentinization, and how they vary as a function of environmental parameters such as temperature, rock composition, and reaction time. Our primary approach is to examine fluid-rock interactions through laboratory experiments, which will supply inputs to geochemical models being conducted by other members of the RPL team and provide a basis for interpreting the distribution and diversity of microbial communities in natural systems. During this first year, experiments were initiated to study fluid-rock interactions in low- to moderate-temperature environments, with initial results expected early in 2016. Also this year, we continued development and construction of a new type of reaction vessel optimized for low temperature (25-100 °C) water-rock reaction experiments.
Project Progress
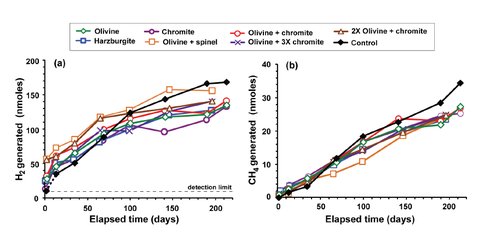
Work in the McCollom lab has focused on the experimental study of the factors that control the rates and amounts of molecular hydrogen (H2) production during serpentinization of ultramafic rocks at moderate hydrothermal temperatures (200 °C), appropriate for shallow submarine settings like that found at the Lost City hydrothermal system on the Mid-Atlantic Ridge. Following up on previous studies (McCollom et al., manuscript submitted to Geochimica et Cosmochimica Acta), initial experimental work is aimed at evaluating the impact of pH on rates of serpentinization and hydrogen generation. To achieve sufficient reaction progress, these are long-term experiments lasting up to a year, so the first results of the experiments are expected to become available in early 2016. Preliminary results suggest that increasing pH can substantially increase reaction rates. We also completed a preliminary study to examine the production of H2 and methane (CH4) during hydrothermal reaction of ultramafic rocks at 90°C, but concluded that reaction rates were too slow to produce significant rock alteration or detectable amounts of H2 or CH4 at laboratory timescales at this temperature.
Work by Templeton/Mayhew has focused on development of a reaction vessel optimized for low (25-100 °C) temperature water-rock reaction experiments. Low temperature experiments have typically been conducted in borosilicate vials sealed with butyl rubber stoppers to maintain anoxic conditions. However, even at low temperatures, the borosilicate vials undergo dissolution and release dissolved silica (SiO2,aq) to solution, which can influence which secondary mineral phases precipitate. During experiments, the rubber stoppers can degrade, resulting in a background H2 concentration that is detectable in mineral-free control experiments. Considering that only very low concentrations of H2 are expected to be produced from water-rock reactions within this temperature range, high background levels of SiO2,aq and H2 present a significant problem for experimental design. Thus, we are working to develop and optimize a reaction vessel constructed entirely of non-reactive titanium, with the capability of repeated sampling of gases, fluids, and minerals. In Year 1 of the RPL NAI, we refined previous iterations of this reaction vessel with modifications to the seals and valves to solve problems with gas and fluid leaks. Currently, we have 2 vessels each with 20 ml and 60 ml volumes in working order, and will employ these in low-temperature experiments during the second year of the project.
To date, we have conducted several experiments to assess the reactivity of partially-serpentinized peridotite with seawater, rainwater, and hyperalkaline fluids, using the borosilicate vial approach. Our key metric is the extent of hydrogen generation at near-surface temperatures from 30-100oC. No measureable H2 generation occurred at the lowest experimental temperatures. However, the fastest rates of H2 production ever measured from ultramafic rock substrates under low-temperature conditions were observed in experiments at 100oC. Moreover, these high rates of hydrogen generation were sustained for several months in replicate experiments. Follow-up experiments also demonstrated that extensive H2 generation at 100oC can be stimulated across a broad range of pH (from pH 8 to >11). Therefore we are conducting several mineralogical and spectroscopic analyses of the rocks before and after water/rock reaction, in order to identify the key mineral reactants and products giving rise to elevated H2 production. These analyses will be complete in early 2016. Future experiments under these experimental conditions will then be conducted in the new reaction vessel design.
Publications
-
McCollom, T. M., & Donaldson, C. (2016). Generation of Hydrogen and Methane during Experimental Low-Temperature Reaction of Ultramafic Rocks with Water. Astrobiology, 16(6), 389–406. doi:10.1089/ast.2015.1382
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Lisa Mayhew
Co-Investigator
Hannah Miller
Co-Investigator
Alexis Templeton
Co-Investigator
Chris Donaldson
Collaborator
-
RELATED OBJECTIVES: