2015 Annual Science Report
 NASA Ames Research Center
Reporting | JAN 2015 – DEC 2015
NASA Ames Research Center
Reporting | JAN 2015 – DEC 2015
Executive Summary
The Ames CAN-7 team seeks a greater understanding of the chemical processes that occur at every stage in the evolution of organic chemical complexity from quiescent regions of dense molecular clouds, through all stages of disk and planet formation, and ultimately to the delivery of materials to planetary surfaces by comets, asteroids, cosmic dust, and meteorites. The overall goal of the effort is to assess the role that astrophysical and astrochemical processes have in the creation of habitable planets and delivery to their surfaces of materials that can play a role in the formation of life.
The team is structured as a coherent program consisting of four well-integrated research projects:
Modeling and Observation of Protostellar Disks
Modeling and Observation of Exoplanets
Laboratory Studies
Computational Quantum Chemistry
A brief description of each of these projects is provided below. Further details about the projects can be found in their individual report sections. We also describe how these projects interact to enhance our overall efforts and how we interact with other NAI Teams, outside organizations, and space missions to enhance our efforts.
Modeling and Observation of Protostellar Disks
This project focuses on modeling the chemical evolution that occurs during the protoplanetary disk stage of planetary system formation. Disk matter provides the raw material for planet formation and its composition bears directly on the composition of the planets and the origin of life on them. We study disk chemical evolution via a two-pronged approach: (i) theoretical modeling of disk physical structure and its chemistry in time and the transport of matter in the disk as it evolves, and (ii) constructing synthetic line and continuum spectra and images of gas and dust in disks to compare with observational data. New chemical networks incorporating results from the Laboratory and Quantum Calculation projects are being developed and disk model results compared with telescopic data to infer conditions under which planets form.
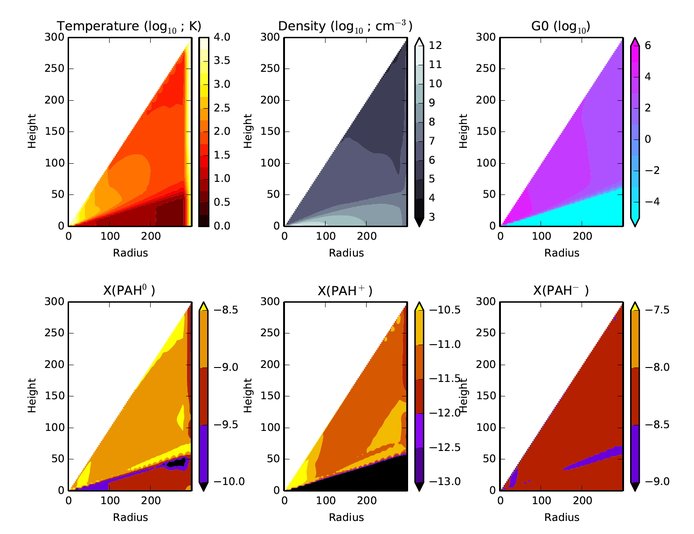
Over the past year we have concentrated on modeling PAH (polycyclic aromatic hydrocarbon) emission from disks around intermediate mass Herbig stars and low-mass T Tauri stars (young solar-type objects) to discriminate between two proposed mechanisms to explain the high rates of detection of PAH features in Herbig stars and their non-detection in T Tauri stars: (i) a depletion of PAHs, or, (ii) a lower excitation rate of PAHs due to low UV fields in low mass stars. We made use of existing thermochemical disk models to compute the abundance of PAH cations, anions, and neutrals in a chemical framework in a self-consistent dust collisional coagulation/fragmentation equilibrium model (Figure 1). Model output results were fed into the Ames PAH Spectral Database to compute a composite disk spectrum for comparison with observations of real disks. Monte Carlo techniques were used to co-add the infrared emission from dust with the PAH emission features from the database for each model run.
Our modeling shows that the non-detection of PAHs in T Tauri disks is consistent with PAH depletion factors of >100 from the interstellar medium. We also found that the strength of the UV field strongly influences the detection of PAH features – a 10-fold drop in UV causes a significant decrease in the excitation and subsequent infrared emission of the PAHs. This work allows for more robust detection and interpretation of PAH abundances in planet forming disks.
Modeling and Observation of Exoplanets
This project uses the Doppler velocity technique to detect and characterize extrasolar planets and uses accumulated exoplanet data to establish the nature and diversity of planetary systems in the galaxy, with an emphasis on establishing the abundance of habitable planets in the universe. The work done in the past year involved theoretical studies of planet formation and evolution, and direct planet detection around bright nearby stars using the new Automated Planet Finder (APF) telescope on Mt Hamilton. Highlights include the discovery of a remarkable 6-planet system orbiting the sun-like star HD 219134. The low-mass innermost planet of the HD 219134 system has been observed to transit the host star and will be an important target for the James Webb Space Telescope (JWST).
One of our more interesting results is the discovery that “true” Jupiters – and by extension, our own Solar System – are rare. Of 1,122 stars studied, only 8 had planets with Jupiter-like masses (0.3-3 times that of Jupiter) and orbital periods (0.5-2 times that of Jupiter). At most, 3% of Sun-like stars host planetary systems having a fundamental resemblance to our own. While this result does not tell us the frequency of Earth analogs, it is clear that the variety of planetary systems is far larger than what we would guess from our Solar System.
Laboratory Studies
Members of the Laboratory Studies project conduct experimental studies with mixtures of minerals, organic molecules, and ices to examine radiation- and surface-mediated chemistry under astrophysically relevant conditions. We concentrate on environments involving low temperatures, low pressures, and high radiation fields. Experiments are designed to characterize organic products and compare them to organics found in protoplanetary disks, exoplanets, meteorites and cosmic dust, and samples from sample return missions. During the past year our efforts have concentrated on catalytic chemistry that occurs at gas-solid interfaces and on the production of organic molecules in mixed molecular ices exposed to ionizing radiation. Specific classes of compounds we investigated include nucleobases, heterocyclic aromatic hydrocarbons, sugars and sugar-related compounds, and N-rich organics made in simulations of Pluto’s surface.
We have acquired a Harrick Praying Mantis Diffuse Reflectance apparatus and corresponding low temperature cell and successfully interfaced them with one of our existing vacuum systems and infrared spectrometers. This apparatus will be used to study the adsorption processes of polycyclic aromatic hydrocarbons (PAHs) and ice mixtures onto mineral grain surfaces and study any catalytic properties the grains may have when irradiated with high energy UV photons. We have also studied the photochemistry of mixed molecular ices and demonstrated the production of (i) the nucleobases adenine and (probably) guanine, (ii) aromatic heterocycles from polycyclic aromatic hydrocarbons (PAHs), and (iii) sugars and sugar-related compounds (Figure 2). In preparation for the flyby of Pluto by the New Horizons spacecraft, we also carried out experiments using UV photons and energetic electrons to study the irradiation of Pluto ice analogs. These experiments demonstrated that the surface of Pluto probably contains N-rich, highly polymerized organic materials. The infrared spectra of these materials will be compared to the spectra of Pluto returned by the New Horizons spacecraft.
Computational Quantum Chemistry
We use computational quantum chemistry techniques (ab initio and density functional approaches) to elucidate the detailed mechanisms of chemical reactions both in the gas and condensed phases, as well as diverse catalytic processes. The quantum chemistry results (reaction mechanisms, reaction rates, and product ratios) are then used to help interpret laboratory results and feed into improved parameters for the disk modeling studies.
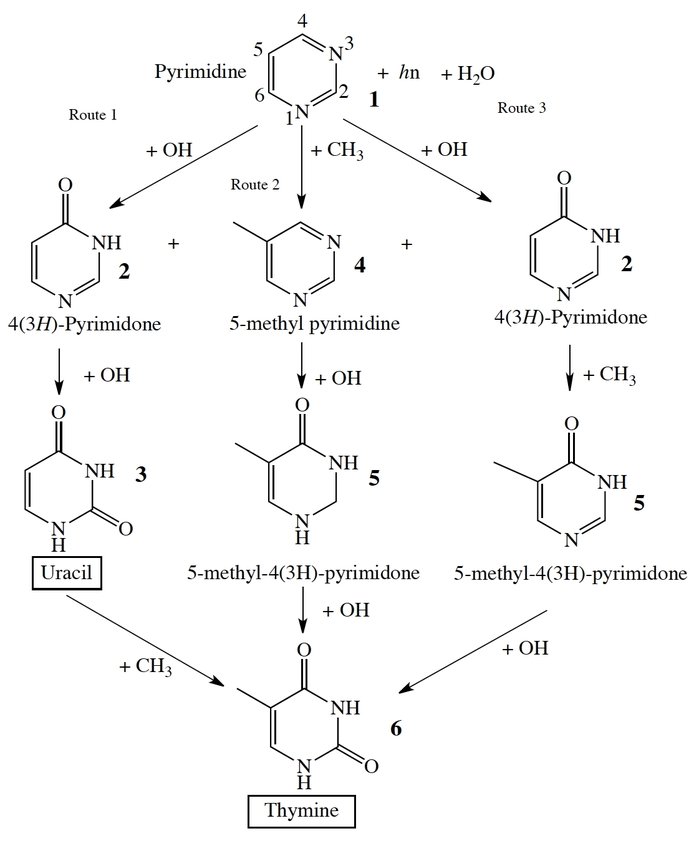
This past year we investigated the chemistry of nitrogenated PAHs. We showed that dihydroisoquinoline – a nitrogen substituted polycyclic aromatic hydrocarbon – can be synthesized in the cold dark molecular clouds, which suggests that the formation of nitrogenated PAHs and related biomolecules in interstellar molecular clouds could be common. Further work concentrated on formation of the nucleobase thymine in astrophysical ice analogs containing pyrimidine. Quantum calculations showed that thymine (Figure 3) can be produced, but with only very limited efficiency and its production requires the presence of an H2O matrix. These results suggest abiotically produced thymine may have had a limited role in prebiotic chemistry. Work is currently in progress to estimate the efficiency of the production the purine-based nucleobases adenine and guanine.
![Figure. 2. Sugars, sugar alcohols, sugar acids, and dicarboxylic sugar acids found in the Murchison and Murray meteorites [4]. We searched our residues for compounds in the first 3 columns and those](../../../../media/medialibrary/2016/02/fig2_i76dHyE.jpg)
Significant progress was also made calculating the spectroscopic properties of small to medium sized molecules. A framework for calculating the anharmonic vibrational spectra and rovibrational spectroscopic constants of PAHs was developed and tested in collaboration with members of the Dutch Astrochemistry Network (DAN). The study focused on naphthalene, anthracene, and tetracene and obtained excellent results. Additionally, the possible importance of charged molecular clusters like OCHCO+ and NNHNN+ in astrophysical environments was investigated. State of the art electronic structure methods were employed to investigate their potential energy surfaces and to predict their rovibrational spectroscopic constants and spectra.
Interactions Between the Individual Programs
Our NAI Team is designed to benefit from close interactions between the experimental chemists, quantum chemists, astronomers, and astrophysicists working on the different projects. For example, the results of the Disk Modeling project provide members of the Laboratory Studies project with calculated physical parameters (temperatures, pressures, compositions, etc.) of protostellar disks that define the conditions to be reproduced in experiments. In return, the experiments provide empirical data – information on bonding, adsorption and desorption rates, degradation rates, chemical species, etc. – that can then be put into the disk models.
Similarly, the quantum mechanical modeling provides insights into the chemical pathways leading to products seen in the laboratory experiments, predicts preferred reaction mechanisms, and predicts the formation of additional products that can then be searched for in the laboratory samples. Similarly, the experimentalists provide chemical observations to the theoretical chemists that can be used to verify their results and aid the refinement of their calculations. Using the laboratory results as checks permits the quantum calculations to be extended to environments beyond the abilities of the laboratory but that are of interest for input parameters for the disk modeling effort.
Interactions of the Team with other NAI Teams and Outside Organizations
Our involvement in the NAI has lead to the creation of many new collaborations, both within and outside the NAI, as well as enhanced several pre-existing collaborative efforts.
During the course of 2015 we helped develop an NAI Synergy Theme that involves close interactions with members of the GSFC and MIT NAI Teams. We will be studying the chemical and physical properties of amphiphiles made in our ice photolysis residues and comparing them both to modern lipids and the amphiphilic molecules found in meteorites. Team member Michel Nuevo was awarded an NAI DDF to assist with this effort.
Members of the Space Sciences and Astrobiology Division at NASA ARC have a long history of collaboration with members of the Dutch Astrochemistry Network (DAN), which is part of the Netherlands Organization for Scientific Research (NWO). Over the past year we have worked to formalize this collaboration through a Non Reimbursable Space Act Agreement (NRSAA) with the DAN. This partnership pursues research on the origin and evolution of molecules in space, particularly in regions of star and planet formation. The two groups participate in joint telecons and meet annually through site visits, sometimes as part of ancillary meetings at other scientific conferences. The 2015 meeting between the groups was held during the March ACS meeting in Denver, CO. This collaboration has already yielded several publications.
Our Team also worked with a variety of organizations to communicate the excitement of Astrobiology to members of the public and others outside the NAI. Activities include providing public talks at Lassen Volcanic National Park during their Dark Skies Festival, sponsoring summer interns from Langston University to work with our team (Figure 4), and making presentations at Native American summer camps in Oklahoma sponsored by the Chickasaw and Choctaw Nations.
The first offering of the Fall School of Astrochemistry on the West Coast was organized by NAI ARC team members Partha Bera, Michel Nuevo, and Chris Materese and held at the SETI Institute in Mountain View, California on 13-16th October 2015. The school highlighted lectures given by Prof. Alexander Tielens (Leiden University, NL, NAI ARC Collaborator, and DAN member) but also included lectures given by Dr. Lou Allamandola (Collaborator), Dr. Timothy Lee (Co-I), Dr. Scott Sandford (PI), Dr. Andy Mattioda (Co-I), and Dr. Farid Salama. The lectures were designed for young scientists – graduate students and post docs – who work in the broad area of astrochemistry. There were 23 participants from institutions in the San Francisco Bay Area. Details can be found at https://fallschoolofastrochem.wordpress.com.
Finally, while not directly responsible for initiating mission concepts, the ARC Team’s work serves to inform the development and execution of a number of space missions involving (i) sample return from asteroids and comets and (ii) space exposure of organic materials. These connections come from involvement of team members on both missions and mission concepts. For example, Team PI Dr. Scott Sandford has worked and continues to work on missions like NASA’s Stardust Comet Sample Return Mission and OSIRIS-REx (Origins, Spectral Interpretation, Resource Identification, Security, Regolith Explorer) Asteroid Sample Return Mission, and JAXA’s Hayabusa Asteroid Sample Return Mission. He is also the PI on CORSAIR (COmet Rendezvous, Sample Acquisition, Investigation, and Return), a New Frontiers concept for a comet surface sample return mission. Similarly, CoI Dr. Andrew Mattioda has worked on the Expose R2 , OREOCube (ORganics Exposure in Orbit cube), and EXOCube (EXposure of Organisms/Organics cube) exposure experiments flown in low Earth orbit.
Publications
- Cottin, H., Saiagh, K., Nguyen, D., Grand, N., Benilan, Y., Cloix, M., Coll, P., Gazeau, M.C., Fray, N., Khalaf, D., Raulin, F., Stalport, F., Carrasco, N., Szopa, C., Chaput, D., Bertrand, M., Westall, F., Mattioda, A., Quinn, R., Ricco, A., Santos, O., Baratta, G.A., Strazzulla, G., Palumbo, M.E., Le Postollec, A., Dobrijevic, M., Coussot, G., Vigier, F., Vandenabeele-Trambouze, Incerti, S., and Berger, T. (2015) Photochemical studies in low Earth orbit for organic compounds related to small bodies, Titan and Mars. Current and future facilities. Bulletin de la Société Royale des Sciences de Liège, 84: 60-73.
-
Burt, J., Holden, B., Hanson, R., Laughlin, G., Vogt, S., Butler, P., … Deich, W. (2015). Capabilities and performance of the Automated Planet Finder telescope with the implementation of a dynamic scheduler. Journal of Astronomical Telescopes, Instruments, and Systems, 1(4), 044003. doi:10.1117/1.jatis.1.4.044003
-
Cook, A. M., Ricca, A., Mattioda, A. L., Bouwman, J., Roser, J., Linnartz, H., … Allamandola, L. J. (2015). PHOTOCHEMISTRY OF POLYCYCLIC AROMATIC HYDROCARBONS IN COSMIC WATER ICE: THE ROLE OF PAH IONIZATION AND CONCENTRATION. The Astrophysical Journal, 799(1), 14. doi:10.1088/0004-637x/799/1/14
-
Fortenberry, R. C., Yu, Q., Mancini, J. S., Bowman, J. M., Lee, T. J., Crawford, T. D., … Francisco, J. S. (2015). Communication: Spectroscopic consequences of proton delocalization in OCHCO+. The Journal of Chemical Physics, 143(7), 071102. doi:10.1063/1.4929345
-
Gorti, U. (2015). Photoevaporation and Disk Dispersal. Proceedings of the International Astronomical Union, 10(S314), 153–158. doi:10.1017/s1743921315006420
-
Kastner, J. H., Qi, C., Gorti, U., Hily-Blant, P., Oberg, K., Forveille, T., … Wilner, D. (2015). A RING OF C 2 H IN THE MOLECULAR DISK ORBITING TW Hya. The Astrophysical Journal, 806(1), 75. doi:10.1088/0004-637x/806/1/75
-
Mackie, C. J., Candian, A., Huang, X., Maltseva, E., Petrignani, A., Oomens, J., … Tielens, A. G. G. M. (2015). The anharmonic quartic force field infrared spectra of three polycyclic aromatic hydrocarbons: Naphthalene, anthracene, and tetracene. J. Chem. Phys., 143(22), 224314. doi:10.1063/1.4936779
-
Materese, C. K., Cruikshank, D. P., Sandford, S. A., Imanaka, H., & Nuevo, M. (2015). ICE CHEMISTRY ON OUTER SOLAR SYSTEM BODIES: ELECTRON RADIOLYSIS OF N2-, CH4-, AND CO-CONTAINING ICES. The Astrophysical Journal, 812(2), 150. doi:10.1088/0004-637x/812/2/150
-
Parker, D. S. N., Yang, T., Dangi, B. B., Kaiser, R. I., Bera, P. P., & Lee, T. J. (2015). LOW TEMPERATURE FORMATION OF NITROGEN-SUBSTITUTED POLYCYCLIC AROMATIC HYDROCARBONS (PANHs)—BARRIERLESS ROUTES TO DIHYDROQUINOLINES. The Astrophysical Journal, 815(2), 115. doi:10.1088/0004-637x/815/2/115
-
Rowan, D., Meschiari, S., Laughlin, G., Vogt, S. S., Butler, R. P., Burt, J., … Diaz, M. (2016). THE LICK-CARNEGIE EXOPLANET SURVEY: HD 32963—A NEW JUPITER ANALOG ORBITING A SUN-LIKE STAR. The Astrophysical Journal, 817(2), 104. doi:10.3847/0004-637x/817/2/104
-
Theis, M. L., Candian, A., Tielens, A. G. G. M., Lee, T. J., & Fortenberry, R. C. (2015). Electronically Excited States of Anisotropically Extended Singly-Deprotonated PAH Anions. The Journal of Physical Chemistry A, 119(52), 13048–13054. doi:10.1021/acs.jpca.5b10421
-
Theis, M. L., Candian, A., Tielens, A. G. G. M., Lee, T. J., & Fortenberry, R. C. (2015). Electronically excited states of PANH anions. Phys. Chem. Chem. Phys., 17(22), 14761–14772. doi:10.1039/c5cp01354b
-
Vogt, S. S., Burt, J., Meschiari, S., Butler, R. P., Henry, G. W., Wang, S., … Laughlin, G. (2015). SIX PLANETS ORBITING HD 219134. The Astrophysical Journal, 814(1), 12. doi:10.1088/0004-637x/814/1/12
-
Yu, Q., Bowman, J. M., Fortenberry, R. C., Mancini, J. S., Lee, T. J., Crawford, T. D., … Francisco, J. S. (2015). Structure, Anharmonic Vibrational Frequencies, and Intensities of NNHNN +. The Journal of Physical Chemistry A, 119(47), 11623–11631. doi:10.1021/acs.jpca.5b09682