2015 Annual Science Report
 NASA Ames Research Center
Reporting | JAN 2015 – DEC 2015
NASA Ames Research Center
Reporting | JAN 2015 – DEC 2015
Computational Quantum Chemistry
Project Summary
We investigated the formation and functionalization of nitrogen substituted cyclic aromatic molecules such as the precursors of biomolecules, polycyclic aromatic hydrocarbons, and the feasibility of their detection via spectroscopic techniques.
Project Progress
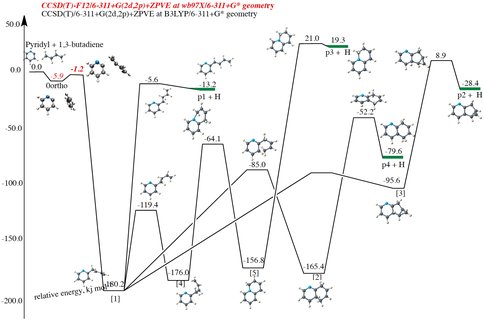
In the first reporting period of this project we have started investigations into the formation of nitrogenated polycyclic aromatic hydrocarbons (PANHs) and the precursors of biomolecules such as purine and pyrimidine, and their functionalization leading to nucleobases in the gas and condensed phases by accurate quantum chemistry methods. In a joint crossed molecular beam experiment and using computational quantum chemistry calculations we showed that PANHs can be synthesized in the cold dark molecular clouds. Dihydroisoquinoline – a nitrogen substituted polycyclic aromatic hydrocarbon – has been synthesized in the laboratory by exploiting a single collision condition between reaction partners pyridyl radical and 1,3-butadiene by our collaborators. By doing high accuracy coupled cluster singles and doubles including perturbative triples (and further including F12 corrections), along with large basis sets we showed that the reaction proceeds via a van der Waal’s complex that circumnavigates the entrance barrier (Figure 1), which indicates that it is plausible at the very low temperatures (10K) found in cold molecular clouds such as TMC-1. These results bode well for further investigations into the origin and identification of nitrogenated PAHs and related biomolecules, many of which are nitrogenated cyclic molecules, in interstellar molecular clouds.
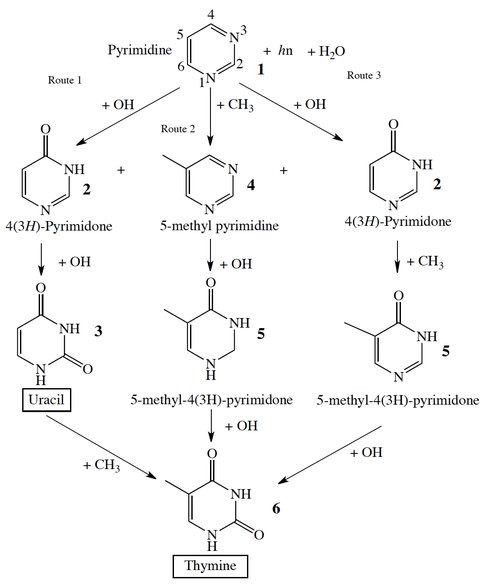
Further work revolved around the investigations of formation of nucleobases thymine (Figure 2), uracil, and cytosine in mixed irradiated astrophysical ices containing pyrimidine. We found by quantum chemistry computation that all three can form in mixed molecular astrophysical ices. However, thymine can be produced by reactions between pyrimidine and hydroxyl and methyl radicals only with very limited efficiency, and only in the presence of a water matrix. The results indicate a limited role for thymine in prebiotic chemistry. Work is currently in progress to estimate the efficiency of similar chemistry producing the purine-based nucleobases, e.g. adenine and guanine.
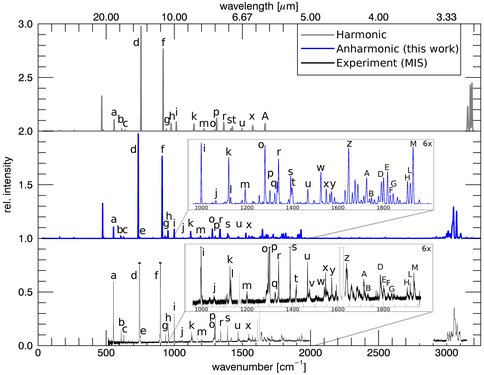
Significant progress was made on the calculation of the spectroscopic properties of small as well as medium sized molecules. A framework for calculating the anharmonic vibrational spectra and rovibrational spectroscopic constants of polycyclic aromatic hydrocarbons (PAHs) was developed, and tested in collaboration with astronomers and experimentalists from the Dutch Astrochemistry Network (DAN). The initial study focused on the “linear” PAH molecules naphthalene, anthracene, and tetracene. As an example of how the calculations compare to experiment for tetracene, see Figure 3. Additionally, the possible importance of charged molecular clusters in certain astrophysical environments was investigated with two studies on the OCHCO+ and NNHNN+ molecular cations. State of the art electronic structure methods were employed to investigate their potential energy surfaces and to predict their rovibrational spectroscopic constants and spectra.
The excited electronic states and electronic spectra of PANH and PAH anions, which may be important in the photo-irradiated regions of the interstellar medium, were investigated. These studies complement our earlier studies of the electronic spectra of PAH and PANH neutral and cationic systems. Anions are relatively rare in most astrophysical environments, but given the size of PAH and PANH molecules, it is known that anions will be more stable and in many cases even support stable excited electronic states. Our studies confirmed these expectations, especially for the PANH molecules.
Publications
-
Fortenberry, R. C., Yu, Q., Mancini, J. S., Bowman, J. M., Lee, T. J., Crawford, T. D., … Francisco, J. S. (2015). Communication: Spectroscopic consequences of proton delocalization in OCHCO+. The Journal of Chemical Physics, 143(7), 071102. doi:10.1063/1.4929345
-
Mackie, C. J., Candian, A., Huang, X., Maltseva, E., Petrignani, A., Oomens, J., … Tielens, A. G. G. M. (2015). The anharmonic quartic force field infrared spectra of three polycyclic aromatic hydrocarbons: Naphthalene, anthracene, and tetracene. J. Chem. Phys., 143(22), 224314. doi:10.1063/1.4936779
-
Parker, D. S. N., Yang, T., Dangi, B. B., Kaiser, R. I., Bera, P. P., & Lee, T. J. (2015). LOW TEMPERATURE FORMATION OF NITROGEN-SUBSTITUTED POLYCYCLIC AROMATIC HYDROCARBONS (PANHs)—BARRIERLESS ROUTES TO DIHYDROQUINOLINES. The Astrophysical Journal, 815(2), 115. doi:10.1088/0004-637x/815/2/115
-
Theis, M. L., Candian, A., Tielens, A. G. G. M., Lee, T. J., & Fortenberry, R. C. (2015). Electronically Excited States of Anisotropically Extended Singly-Deprotonated PAH Anions. The Journal of Physical Chemistry A, 119(52), 13048–13054. doi:10.1021/acs.jpca.5b10421
-
Theis, M. L., Candian, A., Tielens, A. G. G. M., Lee, T. J., & Fortenberry, R. C. (2015). Electronically excited states of PANH anions. Phys. Chem. Chem. Phys., 17(22), 14761–14772. doi:10.1039/c5cp01354b
-
Yu, Q., Bowman, J. M., Fortenberry, R. C., Mancini, J. S., Lee, T. J., Crawford, T. D., … Francisco, J. S. (2015). Structure, Anharmonic Vibrational Frequencies, and Intensities of NNHNN +. The Journal of Physical Chemistry A, 119(47), 11623–11631. doi:10.1021/acs.jpca.5b09682
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Partha Bera
Co-Investigator
Martin Head-Gordon
Co-Investigator
Louis Allamandola
Collaborator
Xander Tielens
Collaborator
Tamar Stein
Postdoctoral Fellow
-
RELATED OBJECTIVES:
Objective 3.2
Origins and evolution of functional biomolecules