2013 Annual Science Report
 VPL at University of Washington
Reporting | SEP 2012 – AUG 2013
VPL at University of Washington
Reporting | SEP 2012 – AUG 2013
Understanding the Early Mars Environment
Project Summary
There is no liquid water on modern Mars, although there is plenty of solid ice. Observations from orbiting satellites and rovers on the ground suggest that liquid water may have flowed over the Martian surface in the distant past. VPL researchers are studying the geologic record of Mars for clues of past water, and investigating climate and chemical conditions under which water would be stable. Team members examined different climate feedbacks and geochemical processes that could have warmed the early Mars. Some members are also active members of the MSL science team.
This year, team members used climate and interior models to demonstrated that broadening of carbon dioxide and water absorption by volcanically-released hydrogen in Mars early atmosphere may have been enough to raise the mean surface temperature of early Mars above the freezing point of water. We also looked for mechanisms that might have produced the abundant perchlorate molecule found on the Martian surface today.
Project Progress
In our early Mars studies on surface environments, Catling and collaborators completed work on understanding the origin and abundance of carbonates on Mars (Niles et al., 2013), and the environmental implications of clay minerals (Ehlmann et al., 2013). Smith, Claire, Zahnle and Catling updated photochemical models of the early Mars environment and completed modeling of the formation of salts from the oxidation of gases in the atmosphere of early Mars. They find that Martian atmospheric chemistry should produce large amounts of nitrate (Smith et al., submitted). Tying in to this, Claire and colleagues measured soluble salts from an interior portion of the Mars meteorite EETA79001, finding perchlorate, chlorate, and nitrate in higher concentrations than nearby ice. Combined with isotopic analyses, this suggests a Martian origin for the salts (Kounaves et al., 2013). This work provided the first measurement of bioavailable fixed nitrogen on Mars, and the second direct measurement of Martian perchlorate. In conjunction with new results from MSL, this implies that perchlorate may be widespread on Mars. Ongoing efforts by VPL team members this year included work on the possibility of oceans on Mars and the formation of salts in the soil.
In MSL work on assessing the habitability of Gale Crater on Mars, Conrad has been engaged in tabulating the dynamics of the surface/atmosphere interface as it relates to the habitability potential at Mars (Mahaffey et al., 2013; Leshin et al., 2013) The measurements made by Curiosity span a range of chemical, physical and morphological attributes that affect the present habitability potential of the planet. In addition the chemical and mineralogical character of one rock unit from the martian past has been measured, and the team has agreed that those data suggest that Mars has had higher habitability potential in its past (Grotzinger et al., 2013; McLennan et al., 2013; Farley et al., 2013). In addition, we have also measured both the radiometric age and the exposure age of that same rock unit, a first on another planet (Farley et al., 2013).
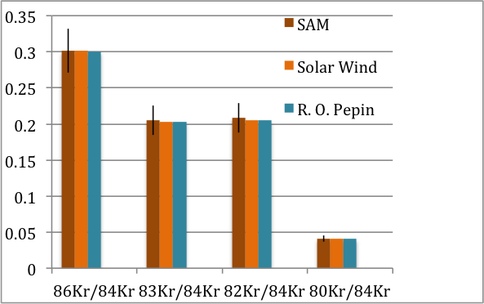
Conrad has also participated in atmospheric chemistry studies of Mars (Atreya et al., 2013; Wong et al., 2013) and in particular taking a lead role in measuring abundance and isotopes of the heavy noble gases, krypton and xenon. The Kr and Xe initial findings were reported at LPSC and AGU and a manuscript is now in prep. Figure 1 shows the SAM measurements in comparison with earlier solar wind measurements.
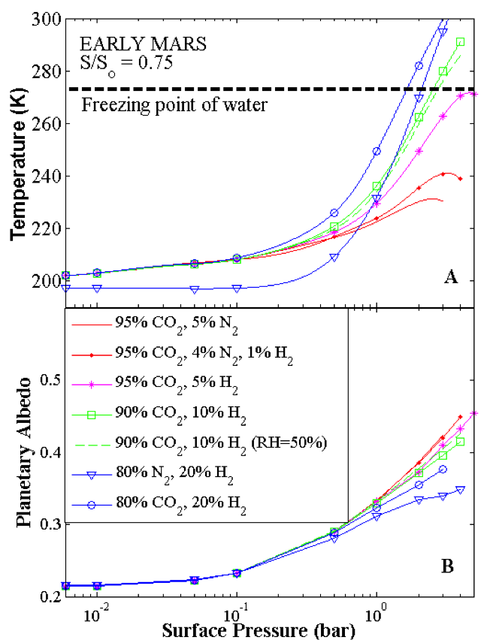
On the theoretical side, Kasting, Ramirez, Kopparapu, Robinson, Freedman and Zugger collaborated on studies of the warming of Early Mars by using CO2 and H2 (Ramirez et al., 2013). They modeled the origin, abundance and lifetime of CO2 in the early Martian environment, and investigated the possible abundance of H2 on early Mars, since H2 can act as a secondary greenhouse gas via collision-induced absorption. They argued that atmospheric H2 abundance should have been high on early Mars. This is due to a number of factors including an extremely reduced mantle for early Mars with would have increased H2 injection into the atmosphere and geometric and the interaction of ionized atmospheric gas with the planets early magnetic field which would have slowed the loss of H2 from the atmosphere. Abundant CO2 may have come from outgassed CO and CH4 from the reduced mantle. Once in the atmosphere, the byproducts of water vapor photolysis would oxidize these species, yielding ~ 5.2 bar CO2 as an initial atmospheric inventory. Vigorous atmospheric escape would have continuously removed this CO2, so recycling and/or continued volcanic outgassing would have been needed to maintain its abundance. Mars’ atmospheres containing 5-20% H2 are a plausible consequence of this volcanic outgassing from a highly reduced martian mantle. With the green house effect from such a CO2-H2 atmosphere these researchers show that Mars could have been warmed above the freezing point of water.
Publications
-
Atreya, S. K., Trainer, M. G., Franz, H. B., Wong, M. H., Manning, H. L. K., Malespin, C. A., … Navarro-González, R. (2013). Primordial argon isotope fractionation in the atmosphere of Mars measured by the SAM instrument on Curiosity and implications for atmospheric loss. Geophysical Research Letters, 40(21), 5605–5609. doi:10.1002/2013gl057763
-
Kounaves, S. P., Carrier, B. L., O’Neil, G. D., Stroble, S. T., & Claire, M. W. (2014). Evidence of martian perchlorate, chlorate, and nitrate in Mars meteorite EETA79001: Implications for oxidants and organics. Icarus, 229, 206–213. doi:10.1016/j.icarus.2013.11.012
-
Leshin, L. A., Mahaffy, P. R., Webster, C. R., Cabane, M., Coll, P., Conrad, P. G., … Moores, J. E. (2013). Volatile, Isotope, and Organic Analysis of Martian Fines with the Mars Curiosity Rover. Science, 341(6153), 1238937–1238937. doi:10.1126/science.1238937
-
Mahaffy, P. R., Webster, C. R., Atreya, S. K., Franz, H., Wong, M., Conrad, P. G., … Moores, J. E. (2013). Abundance and Isotopic Composition of Gases in the Martian Atmosphere from the Curiosity Rover. Science, 341(6143), 263–266. doi:10.1126/science.1237966
-
McLennan, S. M., Anderson, R. B., Bell, J. F., Bridges, J. C., Calef, F., Campbell, J. L., … Moores, J. E. (2013). Elemental Geochemistry of Sedimentary Rocks at Yellowknife Bay, Gale Crater, Mars. Science, 343(6169), 1244734–1244734. doi:10.1126/science.1244734
-
Ramirez, R. M., Kopparapu, R., Zugger, M. E., Robinson, T. D., Freedman, R., & Kasting, J. F. (2013). Warming early Mars with CO2 and H2. Nature Geosci, 7(1), 59–63. doi:10.1038/ngeo2000
-
Wong, M. H., Atreya, S. K., Mahaffy, P. N., Franz, H. B., Malespin, C., Trainer, M. G., … Steele, A. (2013). Isotopes of nitrogen on Mars: Atmospheric measurements by Curiosity’s mass spectrometer. Geophysical Research Letters, 40(23), 6033–6037. doi:10.1002/2013gl057840
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
David Catling
Co-Investigator
Pamela Conrad
Co-Investigator
David Crisp
Co-Investigator
James Kasting
Co-Investigator
Kevin Zahnle
Co-Investigator
Mike Zugger
Co-Investigator
Jacob Haqq-Misra
Collaborator
Tyler Robinson
Collaborator
Megan Smith
Collaborator
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.1
Mars exploration.