2013 Annual Science Report
 University of Wisconsin
Reporting | SEP 2012 – AUG 2013
University of Wisconsin
Reporting | SEP 2012 – AUG 2013
Project 2D: Catalytic Roles of Microbes in Dolomite Crystallization in a Modern Hypersaline Lake
Project Summary
A key question is if the presence of Mg-bearing carbonates, such as dolomite or proto-dolomite, by itself, represents a biosignature. This proposal is based on the observation that inorganic precipitation of Mg-bearing carbonates is difficult in laboratory settings, possibly reflecting the high Mg dehydration energies in aqueous solutions. New work shows that microbial extracellular polymeric substances substance (EPS) from halophylic archaea from a hypersaline lake can catalyze disordered dolomite precipitation at low-temperature. Mg-rich dolomite can form at 40 degree C. No dolomite precipitates from solutions without the biomass. Low-temperature dolomite with wide range of compositions may therefore be used as a biosignature.
Project Progress
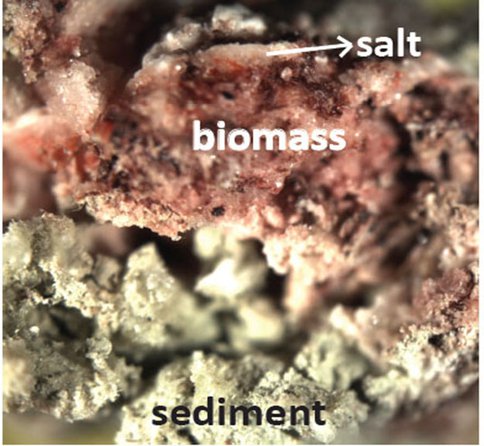
Modern dolomite is observed to form in hypersaline and alkaline marine environments. Such modern dolomite precipitation is commonly attributed to microbial mediation. In order to understand the roles of microbes in mediating dolomite precipitation, we use the biomass collected from Deep Springs Lake in California in dolomite precipitation systems. The biomass in the lake is dominated by micro-organisms of Halorhabdus (archaea, the major one in the biomass), and Halobacteroides halobius. The micro-organisms live between sedeimt mud and top sulfate salt layer (Fig. 1). Halorhabdus cuases red color of the surface the lake.
Our experimental results prove that disordered dolomite can be synthesized at low temperature using the biomass collected from Deep Springs Lake. Based on the glycosyl composition analysis of the biomass, about ten monosaccharides were detected with glucose, ribose and xylose as the most abundant. These bacterially derived extracellular polymeric substances (EPS) might play a crucial role in dolomite precipitation. It is proposed that polysaccharides can be adsorbed on Ca-Mg carbonate surfaces through hydrogen bonding, and weaken the chemical bonding between Mg and water molecules, which can enhance Mg incorporation into carbonate, therefore contribute to the growth of disordered dolomite.
XRD patterns of synthesized HMC (high-magnesium calcite) and disordered dolomite demonstrate that there is a positive relationship between biomass concentration and mole percent of MgCO3 in the precipitates. No dolomite precipitates from solutions without biomass. The Mg2+/Ca2+ ratio from initial solution and temperature also have strong effects on synthesized dolomite based on our experiments. EDS results demonstrate Mg:Ca ratio of precipitates could be as high as 45% under room temperature and 60% under 40°C. TEM images reveal that synthesized dolomites are nano-crystals with similar crystallographic orientations.
Publications
-
Xu, H., & Barton, L. L. (2013). Se-Bearing Colloidal Particles Produced by Sulfate-Reducing Bacteria and Sulfide-Oxidizing Bacteria: TEM Study. AiM, 03(02), 205–211. doi:10.4236/aim.2013.32031
-
Xu, J., Yan, C., Zhang, F., Konishi, H., Xu, H., & Teng, H. H. (2013). Testing the cation-hydration effect on the crystallization of Ca-Mg-CO3 systems. Proceedings of the National Academy of Sciences, 110(44), 17750–17755. doi:10.1073/pnas.1307612110
-
Zhang, F., Yan, C., Teng, H. H., Roden, E. E., & Xu, H. (2013). In situ AFM observations of Ca–Mg carbonate crystallization catalyzed by dissolved sulfide: Implications for sedimentary dolomite formation. Geochimica et Cosmochimica Acta, 105, 44–55. doi:10.1016/j.gca.2012.11.010
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Eric Roden
Co-Investigator
Huifang Xu
Co-Investigator
Minglu Liu
Collaborator
Zhizhang Shen
Collaborator
-
RELATED OBJECTIVES:
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems