2013 Annual Science Report
 University of Wisconsin
Reporting | SEP 2012 – AUG 2013
University of Wisconsin
Reporting | SEP 2012 – AUG 2013
Project 2B: Origin of Carbonates: Environmental Proxies and Formation Pathways
Project Summary
Magnesium isotopes can provide insights into past environmental conditions including formation temperatures and sources of Mg for carbonates, including dolomite, which is a common sedimentary carbonate of the geologic rock record. For Mg isotopes to be a useful proxy, the factors that control isotope fractionation during formation of carbonates must be known. Previous experimental studies have provided conflicting results on potential kinetics effects during the inorganic synthesis of Mg-calcite from solution. To resolve these differences, a matrix of 34 laboratory experiments were conducted to independently determine the effects of temperature, precipitation kinetics, and solution composition (e.g.,pCO2, aqueous Mg/Ca ratio) on Mg- isotope fractionation in the Mg-calcite-aqueous Mg system. Preliminary results suggest that factors in addition to precipitation rate (e.g., aqueous Mg/Ca ratio, pCO2) may play a role in the fractionation of Mg isotopes in the Mg-calcite-aqueous Mg system.
Project Progress
We have been exploring the effects of various experimental parameters on isotopic fractionation, including aqueous Mg and Ca contents, PCO2, temperature, and precipitation rate. This work is aimed at fully exploring the use of ancient carbonates as proxies for paleoenvironments. Carbonates are the most abundant marine chemical precipitate in Earth’s ancient rock record, and carbonates have clearly been identified on the surface of Mars.
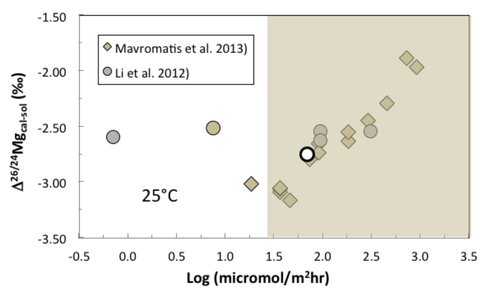
The stable isotopes of C and O in carbonates have been used for decades to infer temperatures of precipitation and biologic “vital” effects. Interpretation of these may be complicated by the fact that, especially for O isotopes, diagenetic or hydrothermal alteration, a significant issue in Precambrian carbonates, may alter the primary C and O isotope compositions. Interest has grown considerably in the fractionation of Mg isotopes in sedimentary carbonate minerals given recent advances in analytical techniques. Understanding the implications of Mg isotope signatures requires a fundamental understanding of the various factors that control isotope fractionation during the precipitation of Mg-carbonates. Toward this end, Li et al. (2012) synthesized Mg-calcite in free drift experiments and determined that Mg isotope fractionation was independent of precipitation rate in the Mg-calcite – aqueous Mg system (circles in Fig. 1). Alternatively, Mavromatis et al. (2013) synthesized Mg-calcite using the chemostat technique and showed that isotope fractionation displayed a pronounced dependence on precipitation rate (diamonds in Fig. 1).
Experimental protocols vary widely between these two studies. In the free-drift experiments of Li et al. (2012), stock reagent solutions were charged with pure CO2 prior to the mixing of experimental solution. Subsequent to mixing, the solutions were bubbled with a CO2/N2 gas mixture (0.038 to 3% CO2) until a metastable state of supersaturation was reached, at which time calcite seed was introduced to promote the heterogeneous growth of Mg-calcite. The precipitation of the solid resulted in a solution composition that drifted toward chemical equilibrium over the course of an experiment. This aspect of free drift experiments confounds the characterization of rate effect because a continually changing fluid composition will result in precipitation kinetics and reaction mechanisms that may vary over the course of an experiment.
To circumvent this problem, Mavromatis et al. (2013) bubbled a MgCl solution with pure CO2 and introduced calcite seed material that partially to wholly dissolved, providing aqueous Ca to the solution. With the introduction of seed material, separate cation (Mg + Ca) and anion (Na2CO3) titrants were pumped continuously into the solution, eventually inducing the heterogeneous (or homogenous?) precipitation of Mg-calcite. A metastable state of supersaturation was eventually achieved when the rate of removal of dissolved constituents through the precipitation of a solid matched the supply of these constituents through the input of tritrants. While solution composition was more highly constrained in these “chemostat” experiments, precipitation rate cannot be accurately constrained because the surface area for Mg-calcite growth was not determined.
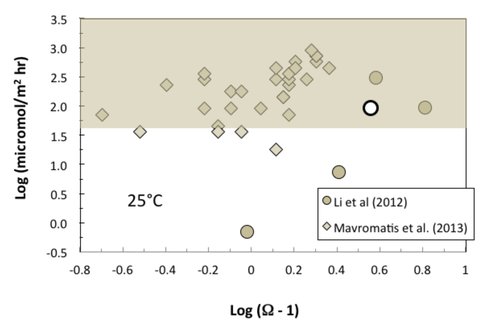
The solution chemistry and rate data from Li et al. (2012) display a positive linear relationship [log rate = 3.046(log(Ω-1)) + 0.006; p = 0.05; n = 5] having a correlation coefficient® of 0.89 (Fig. 2). Unfortunately, these data represent average values for each experiment and this is unlikely to provide insight into how the instantaneous precipitation rates may vary with time, and, critically, how the instantaneous precipitation rates may be related to the instantaneous Mg isotope fractionation factor, given that these variables do not change in a linear fashion over the course of an experiment. On the other hand, the data from Mavromatis et al. (2013) form a linear array [log rate = 0.945(log(Ω-1)) + 2.237; p = 0.002; n = 30] having a correlation coefficient® of 0.54 (Fig. 2). While the statistical probability of a relationship is stronger, the correlation is weaker and the precipitation rate data are unconstrained due to a lack of knowledge regarding the surface area available for the heterogeneous growth of the solid phase.
Additional factors may complicate a comparison of data from these two studies. For example, solution PCO2 was estimated at 1 atm and solution Mg/Ca ratio was < 0.66 for the experiments of Mavromatis while solution PCO2 ranged between ~0.04 and 3.0% and solution Mg/Ca ranged between 3.0 and 13.0 for the experiments of Li. Previous studies have documented that solution PCO2 (e.g., Busenberg and Plummer, 1986) and Mg/Ca ratio (e.g., Mucci and Morse 1983) can strongly influence reaction kinetics for calcite. It is unknown how these variables (and potential others) may influence Mg isotope fractionation in the Mg-calcite-solution system.
Clearly, the results to date demonstrate that the mechanisms behind the large Mg isotope fractionation factors for carbonates are not understood. In an effort to systematically and independently determine the effects of solution composition (e.g., aqueous Mg and Ca content, PCO2), temperature, and precipitation rate on Mg isotope fractionation in the calcite-aqueous Mg system, a matrix of 34 chemostat experiments was conducted between 4° and 45°C, over a range of PCO2 from 0.3 to 3% and solution composition ([Ca]: 3 to15 mmoL; [Mg]: 3 to 300 mmoL) that allows for the factors described above to be evaluated independently for their contribution to Mg isotope fractionation in this system. Mg-calcite was precipitated with up to 12 mol% MgCO3 at precipitation rates (gray areas in Figs. 1 and 2) that span nearly the entire range of rates reported in the literature for synthetic Mg-calcite. The Mg-isotope composition of all aqueous and solid phases is presently being analyzed to develop a better understanding of the salient factors that control Mg-isotope fractionation in Mg-bearing calcite.
References cited
Busenberg E. and Plummer L.N. 1986 A comparative study of the dissolution and crystal growth kinetics of calcite and aragonite. In Studies in Diagenesis (ed. F.A.Mumpton), U.S. Geological Survey Bull. 1578, 139-168.
Li W., Chakraborty S., Beard B., Romanek C.S. and Johnson C.M. 2012 Magnesium isotope fractionation during precipitation of inorganic calcite under laboratory conditions. Earth Planet. Science Lett. 333-334, 304-316.
Mavromatis V. Gautier Q., Bosc O. and Schott J. 2013 Kinetics of Mg partition and Mg stable isotope fractionation during its incorporation in calcite. Geochim. Cosmochim.Acta 114, 188-203.
Mucci A.and Morse J.W. 1983 The incorporation of Mg2+ and Sr2+ into calcite overgrowths; influence of growth rate and solution composition. Geochim. Cosmochim.Acta 47, 217-233.
Publications
- Li, W., Chakraborty, S., Beard, B.L., Romanek, C.S. & Johnson, C.M. (In Preparation). Revisiting the fractionation of Mg isotopes during formation of Mg-calcites. Geochimica et Cosmochimica Acta.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Christopher Romanek
Co-Investigator
Brian Beard
Collaborator
Suvankar Chakraborty
Collaborator
Clark Johnson
Collaborator
Weiqiang Li
Collaborator
-
RELATED OBJECTIVES:
Objective 4.1
Earth's early biosphere.
Objective 7.1
Biosignatures to be sought in Solar System materials