2013 Annual Science Report
 University of Wisconsin
Reporting | SEP 2012 – AUG 2013
University of Wisconsin
Reporting | SEP 2012 – AUG 2013
Project 2A: Magnesium Isotope Fractionation Between Brucite [Mg(OH)2] and Mg Aqueous Species
Project Summary
Recognition of clay minerals on Noachian martian terranes provides important information on the habitability of early Mars. Magnesium isotopic studies can aid in constraining the paleoenvironmental conditions of these clay deposits. Our goal is to conduct Mg isotope exchange experiments between clay minerals and aqueous Mg solutions to better understand how the formation of clay minerals can produce Mg isotope variability. In Mg-bearing phyllosilicates, octahedrally coordinated Mg2+ cations occur in a sheeted structure that is the same as brucite. Determination of Mg isotope fractionation between brucite and aqueous solution, therefore, may provide insight into the origin of Mg isotope variations during weathering and alteration of silicate rocks. Our results document the distinct Mg isotope signals produced by weathering in the presence of organic ligands, raising the possibility that abiotic weathering may be distinguished from biologically-catalyzed weathering using stable Mg isotopes.
Project Progress
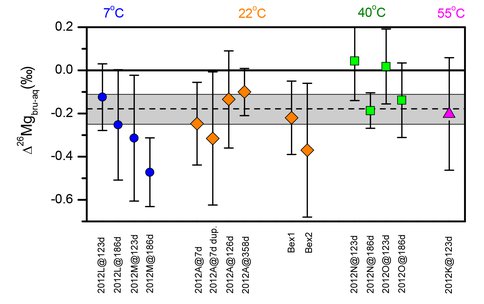
Stable Mg isotope variations, expressed as mass-dependent relations among 26Mg/24Mg and 25Mg/24Mg ratios, are increasingly studied in terrestrial weathering environments, and have great potential for understanding weathering on other planetary bodies such as Mars. Such promise, however, cannot be realized without systematic experimental studies that constrain stable Mg isotope fractionation factors.
We performed Mg synthesis and isotope exchange experiments between brucite and aqueous Mg and brucite and Mg complexed with EDTA. Low temperature (4-55 °C) synthesis and exchange experiments allows us to determine that the fractionation factor between brucite and aqueous Mg (Δ26Mgbrucite-Mg2+) is -0.18±0.07 ‰
Results of MgO hydrolysis experiments in EDTA-bearing solutions suggest that the Δ26Mgbrucite-Mg-EDTA fractionation is ≥ 2.0 ‰ at 22 °C. Based on the Δ26Mgbrucite-Mg-EDTA and Δ26Mgbrucite-Mg2 fractionations, the Δ26Mgbrucite-Mg2+ -Mg-EDTA fractionation is constrained at ≥ +2.2 ‰, suggesting that light Mg isotopes preferentially partition into Mg-EDTA complex relative to Mg aquo ions.
These findings highlight the importance of ligand bond strength in controlling Mg isotope fractionation. To the extent that brucite is a model for Mg isotope fractionation for trioctahedral smectites we note that our brucite experiments would predict that aqueous solutions should have higher 26Mg/24Mg isotope ratios as compared to the clay and this is the opposite of what is observed in natural terrestrial settings where, for example, on Earth, chemical weathering of rocks results in aqueous solutions that have lower 26Mg/24Mg isotope ratios as compared to its catchment lithology. Moreover, the weathered products of the catchment lithology have complementary high 26Mg/24Mg isotope ratios. The origin of these Mg isotope variations is believed to be a result of weathering and the production of clay minerals. These observations may highlight the importance of ligands in controlling Mg isotopic fractionation during weathering. Indeed, significant Mg isotope excursions in clays from Mars may be a useful biosignature if carbonate precipitation can be eliminated as a possibility. Under such a scenario, highly unusual 26Mg/24Mg isotope ratios in weathering products would likely require a role for organic ligands or biology.
Publications
-
Li, W., Beard, B. L., Li, C., & Johnson, C. M. (2014). Magnesium isotope fractionation between brucite [Mg(OH)2] and Mg aqueous species: Implications for silicate weathering and biogeochemical processes. Earth and Planetary Science Letters, 394, 82–93. doi:10.1016/j.epsl.2014.03.022
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Brian Beard
Project Investigator
Clark Johnson
Co-Investigator
Weiqiang Li
Collaborator
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.1
Mars exploration.
Objective 7.1
Biosignatures to be sought in Solar System materials
Objective 7.2
Biosignatures to be sought in nearby planetary systems