2013 Annual Science Report
 University of Wisconsin
Reporting | SEP 2012 – AUG 2013
University of Wisconsin
Reporting | SEP 2012 – AUG 2013
Project 1B: The Extraction of Spiked Amino Acids From a Set of (Clay-Rich) Minerals
Project Summary
In the search for life on Earth and beyond, scientists scan for molecular organic compounds indicative for life, called biomarkers. Amino acids are among the most widespread biomolecules on Earth and play an important role in terrestrial biology by being, among other things, the building blocks of proteins. Thus, they are also selected as priority biomarkers for future life detection missions on Mars. Efficient extraction and detection of these biomarkers is of great importance. It is well known that amino acids degrade over time. This is caused by enzymatic and oxidative processes, as well as by UV- and ionizing radiation from the Sun, unless they are shielded from these influences. Mineral substrates, in particular clays such as montmorillonite, adsorb organic compounds efficiently and may have played a central role in the evolution of life. Rock formations, built up from clay-rich minerals, are therefore a priority target for life detection strategies. However, strong adsorption of amino acids by clay-rich minerals in turn inhibits extraction, resulting in low recovery rates. The aim of this study was to determine the extraction efficiency of amino acids from several distinctive (clay-rich) minerals. This was achieved by spiking minerals with amino acid solutions. After spiking, the samples were subjected to an extraction method. The abundances of recovered amino acids were then compared to the content of the original spiking solutions. Before the extraction experiments were conducted, several parameters were determined that could influence extraction rates (particle size, swelling capacities of the minerals, and carbon/nitrogen content). In this report we discuss preliminary results of adsorption properties of four amino acids: Arginine, Aspartic acid, Glutamic acid and Serine.
Project Progress
In order to determine adsorption characteristics of clays and other substrates, minerals were spiked with a 100 µM L-amino acid mixture containing 17 amino acids for 24 hours. The furnaced Serpentine standard was spiked with ULCMS water as a negative control. After the spiking step, unbound and loosely bound amino acids were washed off in 3 subsequent washing steps and the amino acid content of these supernatants was determined by HPLC/FD. Four amino acids and their adsorption properties to Aluminium and Calcium-pillared montmorillonite were chosen for further analysis for this report, namely: Arginine, Aspartic acid, Glumatic acid and Serine (see Tables 1 and 2).
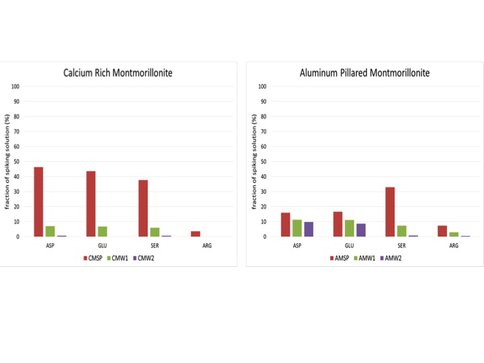
The data show that a large percentage of the originally spiked amino acids is not washed off from the minerals after three washing steps. While the detected amino acid content in the supernatant of the last washing step is almost undetectable for Calcium-pillared montmorillonite it can be detected for the substrate Aluminum-pillared montmorillonite. In the first case this indicates that there are no more loosely bound amino acids present that can be washed away. A large percentage of the spiked amino acids is not detected in the supernatants of the washing steps, indicating adsorption by the mineral.
It is important to investigate the preferences of different amino acids for adsorption to specific minerals (see Figure 1). Both montmorillonites show relatively strong adsorption of Arginine, which has a positively charged side chain. For the Aluminum-pillared montmorillonite it is shown that even after the third washing step, the two negatively charged amino acids Aspartic Acid and Glutamic Acid still persist as loosely bound towards the mineral while the other amino acids appeared to have disappeared after the second washing step. This might indicate the presence of a dynamic process under the influence of water, which is unique for this type of mineral with regard to those amino acids.
Interesting to see is that the presence of Calcium or Aluminum in montmorillonite has a strong influence on the recovery rates and seems to have a selective effect on the adsorption of specific amino acid properties (neutral or positive or negatively charged side chains). Also, the high recovery rates of Aspartic Acid and Glutamic Acid from Aluminum-pillared montmorillonite indicates the presence of a different process, compared to the other mineral that can not only be explained by the presence of montmorillonite but rather be attributed to the presence of Aluminum (or the absence of abundant Calcium). The observation that those amino acids were still detected in the last washing step for this mineral also indicates that different interactions take place. Arginine is most notably extracted with high efficiency from Calcium-pillared montmorollinite. Serine was only detected in very low quantities in all mineral extractions, indicating relatively strong adsorption of this specific amino acid by both minerals. In summary, the results show large variability in extraction efficiencies that appear to result from both properties of the amino acids as well as the characteristics of the minerals.
Publications
-
Direito, S. O. L., Zaura, E., Little, M., Ehrenfreund, P., & Röling, W. F. M. (2014). Systematic evaluation of bias in microbial community profiles induced by whole genome amplification. Environmental Microbiology, 16(3), 643–657. doi:10.1111/1462-2920.12365
-
Frick, A., Mogul, R., Stabekis, P., Conley, C. A., & Ehrenfreund, P. (2014). Overview of current capabilities and research and technology developments for planetary protection. Advances in Space Research, 54(2), 221–240. doi:10.1016/j.asr.2014.02.016
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Pascale Ehrenfreund
Project Investigator
Joost Aerts
Collaborator
Andreas Elsaesser
Collaborator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.