2013 Annual Science Report
 University of Wisconsin
Reporting | SEP 2012 – AUG 2013
University of Wisconsin
Reporting | SEP 2012 – AUG 2013
Project 1A: Detection of Biosignatures in Extreme Environments and Analogs for Mars
Project Summary
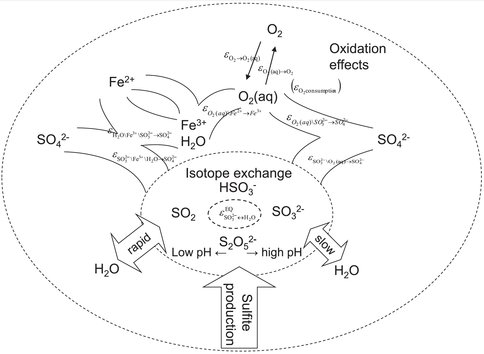
Sulfate, a chemical form containing sulfur and oxygen, is present in ocean water and is a component of minerals on Earth and on Mars, created by evaporation of such water. We have been measuring variations in the relative abundances of naturally-occurring, non-radioactive oxygen isotopes in sulfate to indicate what processes were involved in sulfate formation: for example microbes gaining energy from sulfide or via a non-biological route. A precursor chemical in the formation of sulfate is sulfite, containing sulfur and just a little less oxygen. We have shown recently that the oxidation of sulfite (adding more oxygen), governs the oxygen isotope composition of sulfate. This work will be of significant importance in helping us to understand conditions of formation of ancient minerals.
Project Progress
The Río Tinto area in southwest Spain contains an abundance of sulfate minerals produced by the evaporation of the acid mine waters, which result from microbial oxidation of pyrite. It is a feasible analog for the conditions that existed early in the history of Mars. We have been investigating the use of the oxygen isotope composition of the sulfates as an indicator of the environment which the minerals were formed and, more importantly, as a biosignature of microbial processes.
Most of the reaction pathways for formation of sulfate involved an intermediate sulfite species at some stage. We have continued our collaboration with colleagues at the Max Planck Institute for Marine microbiology in Bremen Germany and have been able to show by means of painstaking laboratory experiments the crucial role played by the isotopic composition of sulfite-oxygen in controlling that of sulfate-oxygen. We measured the oxygen isotope fractionation resulting from abiotic oxidation of sulfite to sulfate either with molecular oxygen or ferric iron and under a wide range of pH conditions, ranging from 1.0 to 13.3. A number of factors are relevant to defining the final isotopic composition and depend on the pH and the oxidant: the dominance of any one of the various isotopic equilibria and how much of the oxygen comes from dissolved gas or water. However, non—equilibrium kinetic isotope effects during sulfite oxidation can also make a contribution to the resultant isotopic composition of sulfate.
Our work explains the observed isotopic fractionations and, in particular, the unusual sense of the fractionation between sulfate and water when ferric iron is the only oxidant. Thus, we can explain our observations of natural processes and validate interpretations.
Publications
-
Müller, I. A., Brunner, B., & Coleman, M. (2013). Isotopic evidence of the pivotal role of sulfite oxidation in shaping the oxygen isotope signature of sulfate. Chemical Geology, 354, 186–202. doi:10.1016/j.chemgeo.2013.05.009
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Benjamin Brunner
Co-Investigator
Max Coleman
Co-Investigator
-
RELATED OBJECTIVES:
Objective 5.3
Biochemical adaptation to extreme environments
Objective 7.1
Biosignatures to be sought in Solar System materials