2013 Annual Science Report
 University of Southern California
Reporting | SEP 2012 – AUG 2013
University of Southern California
Reporting | SEP 2012 – AUG 2013
Life Underground
Project Summary
Our multidisciplinary team from USC, Caltech, JPL, DRI, and RPI is developing and employing field, laboratory, and modeling approaches aimed at detecting and characterizing microbial life in the subsurface—the intraterrestrials. We posit that if life exists, or ever existed, on Mars or other planetary body in our solar system, evidence thereof would most likely be found in the subsurface. This study takes advantage of unique opportunities to explore the subsurface ecosystems on Earth through boreholes, mine shafts, and deeply-sourced springs. Access to the subsurface, both continental and marine, and broad characterization of the rocks, fluids, and microbial inhabitants is central to this study. Our focused research themes require subsurface samples for laboratory and in situ experiments. Specifically, we seek to carry out in situ life detection and characterization experiments, employ numerous novel and traditional techniques to culture heretofore unknown intraterrestrial archaea and bacteria, and incorporate new and existing data into regional and global metabolic energy models.
Project Progress
The centerpiece of our research effort is a set of promising and geologically representative deep subsurface sites on Earth, both continental and marine. These provide samples and opportunities around which we are conducting several comprehensive, interdisciplinary, coordinated, and complementary research efforts. Here, we provide brief summaries of the main accomplishments during this reporting period for five major research themes: (A) access to the subsurface and broad characterization; (B) in-situ life detection and characterization; (C) guided cultivation of intraterrestrials; (D) energy flow and metabolic modeling; and (E) deeply-sourced springs, electrochemistry, and mission relevance.
A. Access to the Subsurface and Broad Characterization.
A key feature of the life underground NAI is the unprecedented access to the subsurface – in both continental and marine settings. This enables a compare and contrast approach to studying life underground. This year, our terrestrial subsurface tasks focused on the Sanford Underground Research Facility (SURF) in South Dakota and one of our “tier-one” boreholes in Death Valley (Nevares DW2). In the marine realm, the key site we have focused on is known as “North Pond”, a sediment “pond” overlying basaltic basement on the western flank of the Mid-Atlantic Ridge in 4400 m water depth.
Our work on the terrestrial subsurface biosphere included site selection, protocol development, and preliminary sampling and analysis. The first site sampled (April, 2013) was Nevares DW-2, with fluids used as primary inocula for guided cultivation exercises described below. Metagenomes from water samples from Nevares DW-2 (as well as from a nearby Death Valley site known at BLM-1) have now been completed, and samples from SURF are now in the sequencing pipeline. It should also be noted that samples collected from all sites received the same general characterizations including: physical measurements (temperature, pH, ORP, dO2, and conductivity); aqueous and nutrient chemistry analysis; metal speciation; dissolved gas compositional and isotopic characterizations (dC and d2D); sulfur isotopes (e.g. d34S of sulfate and sulfide); planktonic cell abundance (flow cytometric and DAPI counts); major organic constituents; electron microscopy; metal speciation; and d2D/d18O. Samples were collected and archived for single cell genomics and DNA extraction, including large filters appropriate for metagenomics.
Significant progress has been made toward solidifying our relationship with SURF. We have produced a memorandum of understanding (MOU) formalizing the relationship between USC and the Facility and are being treated as a general contractor on the site. The Facility published a front-page story describing “Life Underground” in the October SURF Newsletter and one of us (D.P.Moser) has been offered “guide training”, which will enable unescorted access underground. We are currently working on a proposal to participate in a major underground drilling project planned for the “4850-Level” in the Spring of 2014. Our plan is to have members of our team onsite during the entire ~ 1-month, around-the-clock drilling campaign, where we will survey (e.g., spectroscopically) the entirety of the ~ 4,000 lineal feet of planned core and sample segments of special interest (e.g., water/fracture zones) and over a preplanned interval.
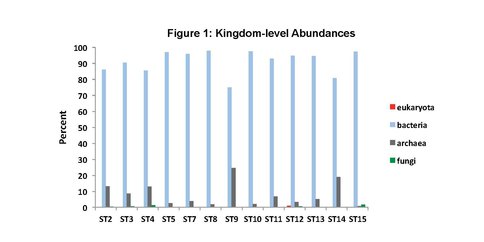
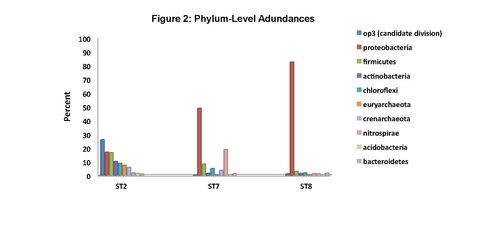
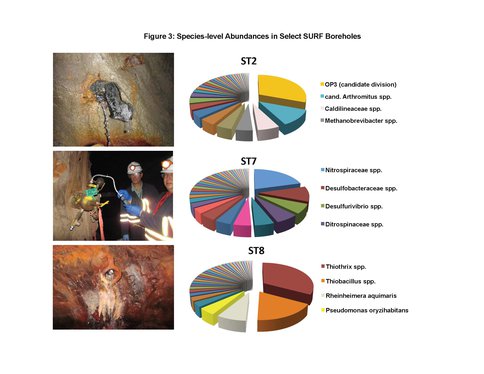
To date, we have surveyed the majority of the ~ 20 mile footprint of the laboratory and sam-pled thirteen flowing legacy boreholes and reference materials (e.g., industrial water). Some aspects of microbial community structure from select SURF samples are shown in Figures 1, 2 and 3. Briefly, Figure 1 shows the overall percentages of life’s three domains, with all samples being dominated by bacteria, to varying degrees. The highest proportions of Archaea (mostly Crenar-chaeota at ~ 10 – 20% of all sequences detected by 16S rRNA gene Illumina sequencing) were from sites with massive accumulations of visible iron oxides. At the phylum-level, the sites varied widely and the relative proportions of the ten most abundant phyla across three representative sample types are shown in Figure 2. The most abundant group in all samples, except for one flowing hole on the 800L, referred to as “Site 2 here”, were the Proteobacteria. This hole (upper panel in Figure 3) is of particular interest because it is dominated (~27% of all life) by candidate bacterial division OP3. Two other holes are representative of other types of sites in the Facility. Here denoted ST7 and ST8, these holes are both on the 4850L. Whereas, ST7 is easily accessed (middle image in Figure 3) and has been excluded from air via a robust, steel casing; ST8 is a flowing hole in a distant, hot part of the Facility (Lower panel in Figure 3) with a massive community of morphologically-conspicuous Thiothrix mat at its outlet (Figure 3). As shown in Figure 3, all three of these type holes had very high diversity but were dominated by handful of abundant but unrelated bacteria; with ST2, ST7, and ST8 being dominated by OP3, Nitrospirae, and g-Proteobacteria (Thiothrix), respectively.
The work we are doing at North Pond integrates geology, geochemistry, and hydrology with recently emergent microbiological, biogeochemical, and observatory science. The major accomplishments as part of this NAI have been to: 1) Establish consistent and reliable methods for extracting DNA and RNA from both rock samples and sediment samples. 2) Initial sequencing of a suite of samples that were selected for analysis based on information gleaned from primary and secondary (alteration) mineralogy. This initial sequencing effort, using traditional Sanger sequencing, is being used to guide the next steps including Tag sequencing and metagenomics. 3) Establishment of subsurface microbiological observatories that can be used by the community involved in subsurface microbiology. These observatories were designed for experiments and analyses 10+ years into the future. Finally, we point out that some resources have been used for analysis of other drilling sites in order to help refine our approaches and for comparison/contrast between the microbial communities harbored in these very different biogeochemical regimes.
B. In-situ Life Detection and Characterization.
In this theme, we aim to detect and characterize in situ life based on spatial distribution of organics (e.g., microorganisms), minerals, elements and isotopes, in the context of textural features, from the macro to the micro scale. The team uses a number of optical techniques ranging the electromagnetic spectrum from the deep UV (<250 nm) to the IR using an array of spectral phenomena such as absorption, Raman scattering, fluorescence, and reflectance for noninvasive means of assessing macro to micro structure, organics, minerals, and elements. These methods help refine samples to smaller targets of interest for analysis by more “invasive” but chemically specific analyses including phylogenetic identification of microorganisms and community structure to elemental/isotopic analysis using techniques such as nanoSIMS.
During this past year our efforts have focused on development of the sample analysis process using a series of lab standards, samples incubated in local field sites operating as “windows” to the subsurface, and subsurface materials already on hand. While this is an ongoing process, initial results demonstrate the uniqueness techniques being used. One technique, deep UV Ra-man/fluorescence, has been under development for the past 15 years as a means to search for organics and habitable regions without the need for sample handling. As such, it has been considered on the Strawman payload for the upcoming Mars 2020 mission. In a similar manner to the stated goals of Mars 2020, the Life Underground team is using the deep UV Raman/fluorescence as a means to detect organics and microbes living in rock such that we can focus detailed mineralogical, structural, and elemental/isotopic techniques. In addition, to better understand the living subsurface communities, we will isolate and attempt to culture detected microbes.
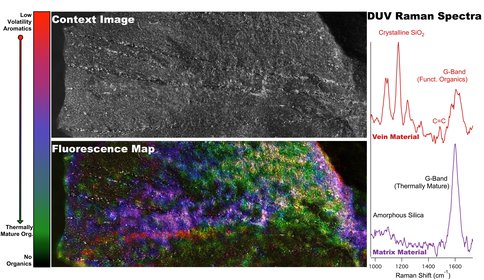
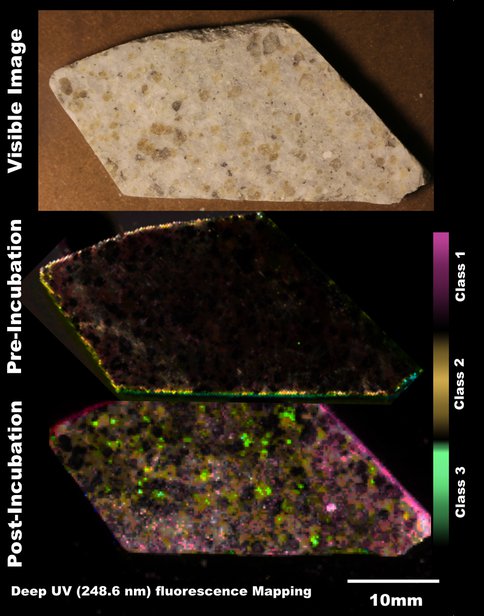
The deep UV instruments are being tested for both laboratory analyses and in situ use at the Life Underground field sites. Prior to site deployments, the team is currently developing the sample analysis process, including the development of a multiinstrument database for Raman, fluorescence, visible images, XRD, GC/MS that merges and organizes data generated for given samples (Figure 4). Figure 4 shows the compilation of contextual imaging, macroscopic deep UV fluorescence, and microscopic deep UV Raman/fluorescence analysis of well-characterized samples from the Archean Fig Tree formation. The results correlate to known chemical features in the sample, however, the data highlight the in situ, spatially preserved capability of the technology to show there are two sources of organics, one more mature and thermally stressed, and the other, a secondary organic that is slightly younger, less heated, likely introduced by an aqueous process.
In addition to the Fig Tree sample, we have also used these instruments to analyze mineral chips that had been incubated for 3 months in The Cedars, a highly alkaline (pH 11) cold spring being fed by serpentinzing sources. These microbially colonized mineral chips provided a test bed for running through the non-destructive and destructive analyses prior to our subsurface FLOC deployments in wells and at the Sanford lab. An example of our analysis pipeline is shown in Figure 5 where a brucite sample was cleaned and scanned by deep UV fluorescence and visible Raman prior to incubating at The Cedars, and then reanalyzed upon collection. Detailed analyses are still in progress, however preliminary data show the development of a biofilm after incubation, which was not visible to the naked eye, but readily observed by deep UV fluorescence. Differentiation correlates with mineralogical variation in the original brucite sample. After initial analyses are complete, we will be transitioning the samples from the optical methods to both micro/nanoscale analyses and culturing methodologies being developed by other team members.
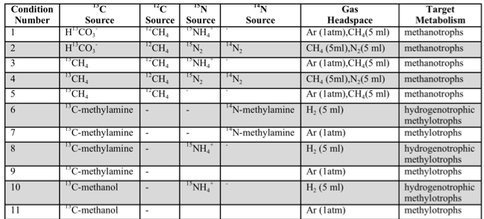
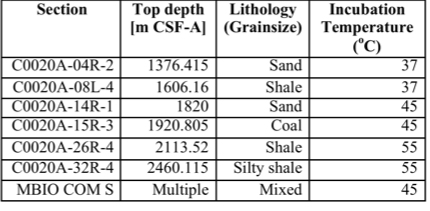
In tandem with sample analyses from The Cedars and Fig Tree, Caltech graduate students Trembath-Reichert, Case, and Marlow have been involved in sample collection and experimental set up for our NAI proposed deep subseafloor sites. Caltech student Elizabeth Trembath-Reichert sailed on IODP Expedition 337 from July-Sept. 2012 and worked with NAI collaborating partners from JAMSTEC in Japan (Inagaki and Moreno) to prepare stable isotope incubations (13C, 15N and D/H) from 4 different lithologies ranging from sandstone to coal (Table 1, Table 2, and Figure 6). The chosen study area for expedition 337 had a high probability to retrieve core from deeply buried coal beds of low thermal maturity, increasing the potential to find deep life by exploring an environment that could utilize coal deposits for carbon and potentially other essential nutrients. Initial analyses of 13C enrichment in the DIC pool after 4 months indicates active microbial respiration in the >2 km coal bed samples with the addition of 13C-methane and select organic carbon substrates. Samples will be harvested after another 3-4 months of incubation to allow additional growth and the run through the deep UV Raman, FISH and nanoSIMS analysis pipeline to further test our methods with a broad range of lithologies. Laboratory incubations of sterilized carbonates (dolomites and calcites) and core material from Death Valley borehole BLM-1 have also been established in three hanging sponge bioreactors housed at Caltech (methane-based, 8ºC) and USC (iron- and manganese-based, 25ºC) to enable further optimization and validation of the sample analysis pipeline.
C. Guided Cultivation of Intraterrestrials.
Developing and harnessing new cultivation techniques represent a major focus of our NAI project. In contrast to traditional growth in well-stirred vessels, which commonly results in the majority of resident archaea and bacteria appearing ‘unculturable’, our team is focused on mimicking the complex interactions and energetic gradients present in the NAI field sites in order to reveal the full diversity of subsurface microorganisms. During this reporting period, our progress on the cultivation front was as follows:
Down-flow Hanging Sponge Reactors for investigating microbial communities in porous media. We constructed, tested, and operated down-flow hanging sponge (DHS) bioreactors under two conditions for enrichment of (i) Metal (Fe/Mn) reducing bacteria and (ii) Mn oxidizing bacteria or Thaumarchaeota under microoxic conditions (Amend Lab). The DHS technology was transferred to our team members (Amend, Orphan) from our JAMSTEC collaborators (Imachi, Inagaki, Moreno). These bioreactors were inoculated from our preliminary sampling project at Nevares Deep Well 2 (Death Valley, CA – see ‘Access to the Subsurface’ section). Preliminary results indicate a successful enrichment, as evidenced by biomass measurements, 16S rRNA analysis, and microscopy (Figure 7).
Electrode Cultivation of metal-reducing bacteria from subsurface sites. We developed the first electrode cultivation reactor targeting subsurface microorganisms (Figure 8, El-Naggar Lab). The bioreactor was completed in May 2013, and has been inoculated with Nevares Deep Well 2 metal-reducing bacteria from the DHS enrichment described above. Four redox conditions are being maintained simultaneously using carbon cloth working electrodes (50 mV, 150 mV, 250 mV, and 350 mV vs. Ag/AgCl) using a 4-channel potentiostat purchased with NAI funds. Preliminary electrochemical, microscopy, and molecular biology analyses point to the growth of two metal reducers (Rhodoferax ferrireducens and another unknown organism) at the higher working potentials of 250 mV and 350 mV vs. Ag/AgCl.
On chip Cultivation. We designed and microfabricated the ‘on chip’ cultivation platform described in the NAI proposal (Figure 9, El-Naggar lab). This combined electrochemical and microfluidic platform is the first technology of its kind intended for both cultivation and live electron transfer (respiration) measurements between subsurface organisms and microelectrode arrays that will mimic the energetics of mineral surfaces. The system is currently under preliminary testing, and will be inoculated with existing samples from two NAI sites: Nevares Deep Well 2 (Death Valley, CA), and the Sanford Underground Research Facility (Lead, South Dakota).
Components and Mechanisms of Extracellular Charge Transfer in Subsurface Microbes. Dr. Yuri Gorby recently established a state-of-the-art laboratory at Rensselaer Polytechnic Institute after accepting a faculty position in the Department of Civil and Environmental Engineering. In addition to attracting undergraduate and graduate researchers, he instrumented his laboratory with chemostats, biofilm reactors, microscopes, and multichannel potentiostats necessary for the NAI team’s research focus on the components and mechanisms of extracellular charge transfer in subsurface microbial communities. At USC, Dr. El-Naggar and his collaborator Dr. Urbashi Mitra developed a new stochastic model for electron transfer in subsurface bacterial ‘cables’ responsible for coupling spatially-separated biogeochemical processes in marine sediments (publication in review).
D. Energy Flow and Metabolic Modeling.
All life requires energy. And that energy is ultimately harvested through the catalysis of electron transfer (redox) reactions. The amount of energy that is available from these reactions determines how fast microorganisms grow and thus the rate and quantity of biomass produced in a given setting. In this research theme, we use numerical energy modeling to map out habitability on a local, regional, and global scale—on Earth and Mars. Several projects were carried out as part of this theme, including the calculation of reaction energetics of organic synthesis inside and outside the cell, modeling of microbial reaction rates in a hydrothermal vent chimney, catabolic energy as a function of depth in marine sediments, and the potential for microbial mediation of anaerobic oxidation of methane (AOM) on Mars.
In Amend et al. (2013), an array of organic synthesis modeling studies were reviewed. The biogeochemical scenarios evaluated included those in present-day hydrothermal systems and in putative early Earth environments. It was shown that the formation of relatively simple organic compounds and biomolecules can be energy-yielding at conditions that occur in hydrothermal systems. A new approach for estimating the energetics of polymerization reactions—specifically those associated with polypeptide formation from amino acids—was also presented.
In LaRowe et al. (2014), a fully coupled biogeochemical reaction-transport model of a hydro-thermal vent chimney was developed that explicitly quantifies the rates of microbial catalysis while taking into account geochemical processes such as fluid flow, solute transport, and redox reactions associated with fluid mixing as a function of temperature. The considered metabolisms included methanogenesis; aerobic oxidation of hydrogen, sulfide, and methane; and sulfate reduction by hydrogen and methane.
In LaRowe and Amend (2014), standard Gibbs energies and chemical composition data are combined to constrain likely metabolic strategies and rates in marine sediments at several sites. In conjunction with cell count data, energy calculations in the sediments at South Pacific gyre, the Peru margin, and the Juan de Fuca ridge flank illustrate the importance of normalizing energy availability to the limiting substrate and how geochemical data can be used to better understand the distribution of life deep in marine sediments.
In Marlow et al. (2014), seven distinct fluids representative of putative martian groundwater were used to calculate Gibbs energy values for AOM in the presence of dissolved methane under a range of atmospheric CO2 partial pressures. In all scenarios, AOM is exergonic. A reaction transport model was then used to examine how environmentally relevant parameters such advection velocity, reactant concentrations, and biomass production rate affect the spatial and temporal dependences of AOM reaction rates.
E. Deeply-sourced Springs, Electrochemistry, and Mission Relevance.
In this theme, we focus on life in deeply-sourced springs: how to detect it, how to cultivate it, how it survives and grows (physiology), and its genetic/genomic properties. Our approach is three-fold, with field work at a site called The Cedars, lab work on energy flow and electrochemistry, and the development of instrumentation for life detection: these are described briefly below. (1) At the Cedars subsurface water and associated microbes are “delivered” to the surface by the natural flow of subsurface water. The interest lies in the fact that these conditions are so extreme that we expected no life to be present – yet we have found an abundance of types and numbers of microbes. In addition to this basic science interest, we want to characterize the types of organisms, given the strong interest these days in serpentinization sites as potential sites for the origin and early evolution of metabolism and life. (2) A second part of the project involves the study of extracellular electron transport (EET) as a mode of metabolism in energy-limited environments. This has expanded to using solid surfaces (minerals and charged electrodes) as either electron acceptors for respiration, or as electron donors (energy sources). This work has led to new techniques for the isolation of microbes, and to new methods of recycling and biore-mediation. (3) An ancillary, but important part of our project team is our connection with NASA missions. Dr. Nealson is a member of the Mars Science Team involved with data analysis and presentation for the MSL (Curiosity) mission. In addition, he is a member of the science team for an ASTID proposal for fabrication of an instrument for future Mars exploration (in collaboration with Co-I Bhartia).
We have made major progress in the area of the study of an alkaline ecosystem in Northern California called the Cedars, and the organisms that inhabit this extreme site. This includes an in-depth description of the study site (Morrill et al., 2013), a detailed analysis (using molecular phylogenetic approaches) of the microbial populations present (Suzuki et al., 2013), and some detailed studies of the physiology of bacteria isolated from The Cedars (submitted for publication). These studies have led to the development of new methods for population analysis, using metatranscriptomics (Ishii et al., 2013a, Nature Comm), and the detection of genes activated by changes in surface electrochemical charge (Ishii et al., 2013b, ISME J). In addition, as part of the Mars Science Team, Dr. Nealson has participated in the data analysis and publication of recent articles dealing with the geochemistry of Mars (Mahaffy et al., 2013; Webster et al., 2013; Williams et al., 2013; Blake et al., 2013; Bish et al., 2013; Leshin et al., 2013; Meslin et al., 2013).

-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Jan Amend
Project Investigator
Rohit Bhartia
Co-Investigator
Katrina Edwards
Co-Investigator
Moh El-Naggar
Co-Investigator
Yuri Gorby
Co-Investigator
Duane Moser
Co-Investigator
Kenneth Nealson
Co-Investigator
Victoria Orphan
Co-Investigator
Holly Willis
Co-Investigator
-
RELATED OBJECTIVES:
Objective 2.1
Mars exploration.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.3
Origins of energy transduction
Objective 4.1
Earth's early biosphere.
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.2
Co-evolution of microbial communities
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems
Objective 6.2
Adaptation and evolution of life beyond Earth
Objective 7.2
Biosignatures to be sought in nearby planetary systems