2013 Annual Science Report
 Pennsylvania State University
Reporting | SEP 2012 – AUG 2013
Pennsylvania State University
Reporting | SEP 2012 – AUG 2013
Biosignatures in Ancient Rocks - Ohmoto Group
Project Summary
Ohmoto group’s activities during the fifth year of the project have focused on the redox evolution of the Earth, particularly the evolution of an aerobic world and the changing O2 content of the Archean atmosphere and oceans. This question has been pursued mostly by investigations of: (1) the origins of MIF-S; (2) the behaviors of redox sensitive elements (Fe, S, C, U, Mo, Ce etc) in (a) submarine hydrothermal systems and (b) paleosols, mostly from the Pilbara Craton, Western Australia. We have also investigated the connection between the evolution of the atmospheric pCO2 and that of the earth’s biosphere through pre-biotic, anaerobic, pre-plant aerobic, and plant stages.
Project Progress
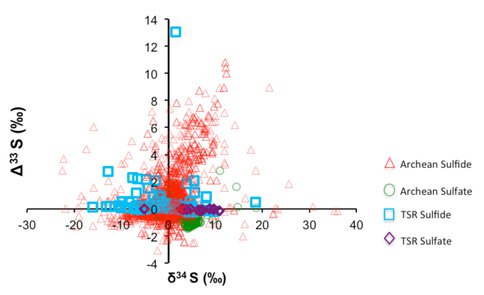
Origins of MIF-S: The presence of MIF-S in some Archean-aged rocks hasbeen considered the strongest evidence for the “Great Oxidation Event” (GOE) at 2.4 Ga. This is because the only known mechanism for MIF-S generation was atmospheric UV reactions of volcanic SO2 in an O2-poor atmosphere. We have been testing an alternative hypothesis of generating MIF-S signatures by thermochemical sulfate reduction using solid organic matter.
Our experiments of thermochemical sulfate reduction using simple amino acid crystals and aqueous sulfate at 150-250°C produced H2S and organic-bound S species with distinct MIF-S signatures (δ34S = 15 to +15‰ relative to SO42; (Δ33S = -0.2 to +2.3‰; and Δ36S/Δ33S = -5 to +5). The experimental MIF-S values overlap with at least 60% of the data on natural samples >2.4 Ga in age (Figure 1). MIF-S bearing minerals predominantly occur in organic C- and pyrite-rich black shales that were hydrothermally altered during diagenesis (Ohmoto, 2006; Ohmoto et al., accepted for publication). These data suggest that MIF-S signatures in both pre- and post- 2.4 Ga rocks could have been created by reactions between solid organic matter (kerogen) and SO42--rich seawater during early stages of sedimentation under the influence of large-scale submarine hydrothermal activities, rather than by UV photolysis of SO2 in an anoxic atmosphere. Therefore, the MIF-S record in rocks may not be linked to the atmospheric evolution, but instead may be linked to the thermal evolution of the early Earth. Thermal and tectonic conditions of the early Earth favored the creation of large anoxic rift basins with high heat fluxes where organic matter could accumulate under SO4-rich seawater. The presence of SO4-rich seawater would imply that the modern-style sulfur cycle, linked to an oxygenated atmosphere, was developed as early as ~3.5 Ga.
Redox-sensitive elements in submarine hydrothermal systems
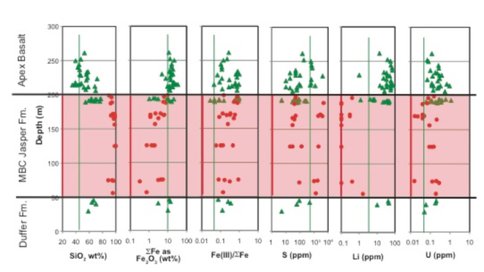
ABDP #1 core: The Archean Biosphere Drilling Project (ABDP) recovered a 260 m-long drill core section (ABDP #1) at Marble Bar, Pilbara, Western Australia. It encompasses one of the world’s oldest (3.46 Ga) jasper formations (i.e., low-grade banded iron formation) and associated submarine basalts. We (Hoashi et al., 2009, Nature Geosciences) reported that the hematite crystals in the jaspers formed at T >60°C from the mixing of Fe2+-rich submarine hydrothermal fluids (T = 150-250°C) and O2-rich deep ocean water. Subsequent research by our group (Ohmoto et al., 2010 & 2011; in prep) has led to a discovery that the jaspers and submarine basalts in ABDP #1 core exhibit significant enrichments of ferric-iron (FeIII), U, Mo, and Li, and positive and negative anomalies in cerium (Ce) concentrations (Figure 2), which are essentially the same characteristics of modern jasperoids and submarine basalts that were formed by reactions with oxygenated deep ocean water. These data suggest that by 3.46 Ga, the atmosphere and oceans were fully oxygenated, that the oceans were Fe-poor, but U-, Mo- and Li-rich, that large continental masses had developed, and that the modern-style geochemical cycles of redox-sensitive elements were established. Through literature survey, we have found that the enrichments of FeIII and U are common in the country rocks around volcanogenic massive sulphide deposits younger than at least ~3.2 Ga. Subduction of FeIII- and U-enriched oceanic crust may have created large-scale heterogeneity of the mantle since ~3.5 Ga, potentially the source of “lead (Pb) paradoxes”, where the mantle contains more radiogenic Pb and higher U/Th ratios than the bulk Earth. Therefore, through the creation of the oxygenated oceans and atmosphere, oxygenic photoautotrophic organisms have influenced the geochemistry of the deep Earth and volcanic systems since ~3.5 Ga.
3.2 Ga Panorama hydrothermal systems: As part of his Ph.D research, Mr. Brainard has modeled iron utilizing thermochemical sulfate reduction based on chemical data from the Panorama District (Pilbara craton, Western Australia) suggesting that the deep-ocean water at 3.2 Ga possessed similar SO42- concentrations to the modern (at least 1mmol, likely greater than 5mmol). The abundance of preserved barites throughout the systems supports this finding of contemporaneous seawater SO42-. In addition to SO42-, this study also found evidence of significant U addition, likely from U adsorption to ferric iron, suggesting that the 3.2 Ga oceans also had U concentrations above what would be expected for the hypothesized anoxic Archean (at least 10 nmol). The redox sensitive nature of both SO42- and U has implications for the O2 content of the ocean and atmosphere, since both are predominantly the product of oxidation reactions.
Experimental simulation of hydrothermal systems: Mr. Ethier (graduate student) has conducted experiments to evaluate the oxidation and co-precipitation of Fe- and Mn-(hydr)oxides. A hydrothermal solution containing soluble Fe2+ and Mn2+ (10-4 and 10-3 M, respectively) is heated and pumped into a reactor containing simulated oxygenated seawater. Parameters such as pH, Eh, conductivity, and DO2 are monitored over time as each species precipitates. Both the produced precipitate and the remaining soluble species of Fe and Mn will be studied for their relative concentrations. Mr. Ethier’s research will aid in understanding how Fe and Mn mineral phases are generated in response to different O2 levels, which is applicable to the generation of banded iron formations.
Redox sensitive elements in paleosols: We have been investigating the behaviors of redox-sensitive elements in a thick (20-100 m) pyrophyllite-rich zone underneath the world’s oldest (~3.4 Ga) erosional unconformity that is traced over 200 km along the strike, from ABDP #8 site to the Strelley Pool – North Pole Dome – Nullagine areas in Eastern Pilbara Craton. Our study, carried out partly as a MS thesis project of Ian Johnson, has revealed that this pyrophyllite-rich zone represents the Earth’s oldest major paleosols, possibly paleolaterites. Similarities in the behaviors of Fe, U and Cr and other elements between this and the 2.2 Ga Hekpoort paleosols suggest that the 3.4 Ga paleosols formed under the influence of soil organisms under an O2-rich atmosphere (Ohmoto et al., 2013, accepted). Results of a preliminary investigation on ABDP #6, which transects the 2.76 Ga Mt. Roe paleosols, especially the behaviors observed in Fe and Cr, also suggest the soil formation under an oxygenated atmosphere.
Detrital kerogen: Matured kerogen in sedimentary rocks generally decomposes back to CO2 with the aid of aerobic organisms during weathering under an O2-rich atmosphere, whereas matured kerogen does not appear to be completely decomposed back to CO2 under a reducing atmosphere even with the aids of anaerobic organisms. Detrital kerogen should be an important component in sedimentary rocks when the atmosphere was reducing. Recycling or non-recycling of kerogen C to CO2 was a major factor affecting the atmospheric _p_CO2 level through geologic time.
Modeling the evolution of atmospheric CO2 through geologic time: A popular solution to the Faint Young Sun Paradox postulates reducing gases like CH4 and H2 were necessary to maintain a warm Early Earth in order to compensate for the reduced luminosity of the early Sun. In a paper submitted to Astrobiology, I have explored a hypothesis that the atmospheric _p_CO2 level has been regulated by the interplay between the mantle dynamics and biology throughout geologic time. Mantle dynamics are responsible for the volcanic CO2 input to the atmosphere-oceans, while biology is responsible for influencing the CO2 output from the atmosphere-ocean by (a) soil formation, (b) burial of organic matter in sediments, and© production of organic haze in the atmosphere. Mantle dynamics have also influenced the size of soil-forming land area. My study, based on the BLAG-type geochemical cycling of CO2, suggests that the atmospheric _p_CO2 fluctuated in response to changes in the nature of biosphere from >1,000 PAL (present atmospheric level) during the pre-biotic stage to ~1000 – 100 PAL during the anaerobic and pre-plant aerobic stages and finally to ~50 – 1 PAL during the vascular plant stage. Throughout the geologic history, CO2 + H2O have been the only greenhouse gases responsible for maintaining the liquid oceans. The transition from an anaerobic to aerobic world was likely to have significantly increased the atmospheric _p_CO2, resulting in warming, not cooling, the Earth, because of more efficient recycling of buried organic C to CO2 during weathering on land under an O2-rich atmosphere. Global glaciation would have occurred during the transition from the pre-biotic world to an anaerobic world at >~3.8 Ga and during the development of the plant world at ~500 Ma because of increased efficiency of CO2 consumption by silicate weathering. Fluctuations in the soil-forming land area profoundly affect the climate of the Earth. Continental breakup would result in a global cooling event, while the formation of supercontinents would result in global warming.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Hiroshi Ohmoto
Project Investigator
Jamie Brainard
Collaborator
Andrew Chorney
Collaborator
William Colon
Collaborator
Lee Kump
Collaborator
Dennis Walizer
Collaborator
-
RELATED OBJECTIVES: