2013 Annual Science Report
 Massachusetts Institute of Technology
Reporting | SEP 2012 – AUG 2013
Massachusetts Institute of Technology
Reporting | SEP 2012 – AUG 2013
Molecular Biosignatures: Preservation in Mineral-Forming Ecosystems
Project Summary
Molecular biosignatures are an important and informative means to reconstruct ancient ecosys-tems, especially, in the case of those dominated by microbes. Most microbes leave only fragmentary chemical records and fossilized hard-parts confined to those taxa with mineralized tests. Preservation can be significantly enhanced when these molecular biosignatures are encapsulated in minerals which are actively forming where the microbes are living and where they may be sequestered from the deleterious effects of oxygen and radiation. We studied several such organo-mineral associations comprising carbonate, silica and gypsum and document a diverse range of molecular biosignatures some of which would be preservable over long timescales. These results are relevant for the study of sediments on the ancient earth, but are also useful in predictive sense for the study of minerals on other planets.
Project Progress
We explored two carbonate depositing systems comprising one shallow water tropical environment and one deep-sea hydrothermal system. The silica-depositing system was a terrestrial hot spring and the gypsum-depositing system was in an endoevaporitic microbial community.
Consistent with their environments of deposition, and concurrent nucleic acid analyses, the biosignatures of shallow water microbial ecosystems included lipids produced by both oxygenic and anoxygenic phototrophs. All of the marine systems, carbonate and sulfate-evaporite, also included biomarker lipids from sulfur-metabolizing microbes. Of particular note was the prevalence of ether lipids in both the deep-water marine and terrestrial hot spring environments. In the process of studying these compounds we documented a new analytical method that can be applied for the analysis of large range of novel membrane lipid types.
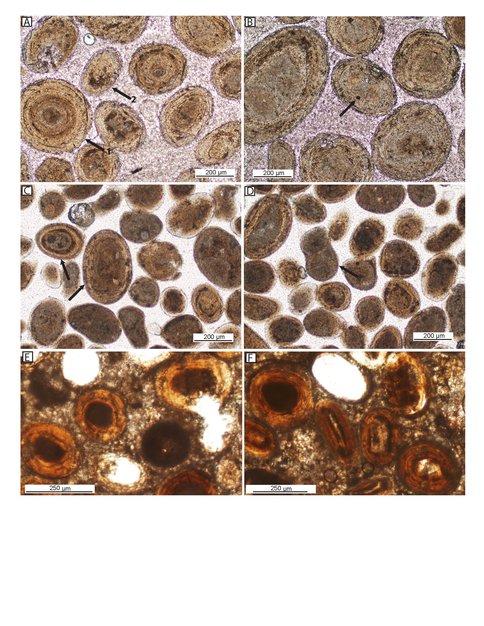
However, our prime focus in this project was to clarify the origin and paleoenvironmental significance of the spherical to ovoid carbonate concretions known as ooids. This is because they are common constituents of ancient carbonate rocks including those of the Precambrian. Of particular note, in this instance, are recent observations that oolitic carbonates preserve C-isotopic records of Neoproterozoic environmental change and carbon cycle anomalies (Kristin Bergmann, PhD Dissertation, Caltech, 2013).
In order to better understand the biosignature preservation potential of oolites, we sought to evaluate the nature of the microbial communities that may or may not play in ooid formation and to examine both extant and ancient oolitic sediments for they types of molecular fossils that would be preserved on geological timescales. We collected samples from beaches and outcrops in the Bahamas and in Shark Bay in Western Australia and found very similar distributions of hydrocarbons, fatty acids and alcohols. A large fraction of these lipids were bound into the carbonate matrix and only released on acid dissolution, which suggests that these lipids were being incorporated continuously during ooid growth. Branched fatty acids, especially 10-MeC16 and 10-MeC17, together with the prevalence of elemental sulfur in the extracts, indicate an origin from sulfate-reducing bacteria. The most enigmatic lipid assemblage is an homologous series of long-chain (C24-C32) fatty acids with pronounced even carbon number preference. C- and H- isotopic analyses indicate a microbial origin, possibly sulfate-reducing bacteria. Lastly, we identified homohopanoic acid and bishomohopanol as the primary degradation products of bacterial hopanoids. The distributions of lipids isolated from Holocene oolites from the Rice Bay Formation of Cat Island, Bahamas were very similar to the beach ooids described above and, in total, these modern and fossil biomarker data lead us to hypothesise that ooids are colonized by a defined microbial community and that these microbes likley mediate calcification. 14C analyses indicated that ooids are formed over thousands of years including periods of carbonate accumulation interspersed with periods of erosion. We intend to continue this work with analyses of oolitic carbonates and ancient hydrothermal silica deposits from deeper in the geological record.
Publications
-
Jahnke, L. L., Turk-Kubo, K. A., N. Parenteau, M., Green, S. J., Kubo, M. D. Y., Vogel, M., … Des Marais, D. J. (2013). Molecular and lipid biomarker analysis of a gypsum-hosted endoevaporitic microbial community. Geobiology, 12(1), 62–82. doi:10.1111/gbi.12068
-
Lincoln, S. A., Bradley, A. S., Newman, S. A., & Summons, R. E. (2013). Archaeal and bacterial glycerol dialkyl glycerol tetraether lipids in chimneys of the Lost City Hydrothermal Field. Organic Geochemistry, 60, 45–53. doi:10.1016/j.orggeochem.2013.04.010
-
Liu, X-L., Summons, R. E., & Hinrichs, K-U. (2012). Extending the known range of glycerol ether lipids in the environment: structural assignments based on tandem mass spectral fragmentation patterns. Rapid Commun. Mass Spectrom., 26(19), 2295–2302. doi:10.1002/rcm.6355
-
Schubotz, F., Meyer-Dombard, D. R., Bradley, A. S., Fredricks, H. F., Hinrichs, K-U., Shock, E. L., & Summons, R. E. (2013). Spatial and temporal variability of biomarkers and microbial diversity reveal metabolic and community flexibility in Streamer Biofilm Communities in the Lower Geyser Basin, Yellowstone National Park. Geobiology, None, n/a–n/a. doi:10.1111/gbi.12051
-
Summons, R. E., Bird, L. R., Gillespie, A. L., Pruss, S. B., Roberts, M., & Sessions, A. L. (2013). Lipid biomarkers in ooids from different locations and ages: evidence for a common bacterial flora. Geobiology, 11(5), 420–436. doi:10.1111/gbi.12047
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Roger Summons
Project Investigator
Steven Beaupre
Collaborator
Laurence Bird
Collaborator
David Des Marais
Collaborator
Aimee Gillespie
Collaborator
Kai-Uwe Hinrichs
Collaborator
Linda Jahnke
Collaborator
Sara Lincoln
Collaborator
D'Arcy Meyer-Dombard
Collaborator
Sara Pruss
Collaborator
Mark Roberts
Collaborator
Florence Schubotz
Collaborator
Alexander Sessions
Collaborator
Everett Shock
Collaborator
-
RELATED OBJECTIVES:
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.3
Biochemical adaptation to extreme environments
Objective 7.1
Biosignatures to be sought in Solar System materials