2013 Annual Science Report
 Massachusetts Institute of Technology
Reporting | SEP 2012 – AUG 2013
Massachusetts Institute of Technology
Reporting | SEP 2012 – AUG 2013
Molecular Biosignatures: Hopanoid Sources in Modern Systems
Project Summary
Molecular fossils preserved in sedimentary rocks provide a record of Earth’s early biosphere and its associated carbon cycle. Among the earliest and most abundant molecular fossils are the hopanoids. Derived primarily from bacteria, their diagenetic products, the hopanes, are detectable over timescales of billions of years and have been proposed to be among the most abundantly preserved molecules on Earth. However, an overall picture of their environmental, physiological, and taxonomic origins remains elusive. Are they primarily remnants of primary producers or of heterotrophic consumers? Do they primarily come from free-living marine communities, or from shallow mats, tidal zone communities, or even terrigenous runoff? Here we aim to obtain compound-specific carbon isotope data for hopanoids to infer their sources in modern systems, as proxies for understanding ancient environments.
Project Progress
Our focus is on the following questions: (1) Are hopanoids in modern marine sediments primarily planktonic in origin, or are they imported as part of the flux of estuarine or terrigenous organic matter? (2) Are most hopanoids the products of primary producers or are they products of the heterotrophic community responsible for organic matter degradation? (3) Is the structural and isotopic diversity of hopanoid lipids related to the abundance and DNA genetic diversity of hopanoid producers in the environment, or are hopanoid structural fingerprints and predicted degradation products mostly diagenetic? To answer these questions we are investigating compound-specific isotopic compositions of various functionalized BHPs extracted from marine core-top sediments from a range of environmental settings, and climatic and depositional gradients. We will compare the BHP isotopic compositions with published latitudinal patterns of Δ14C and δ13C for terrestrial biomarkers, and δD values for meteoric water and seawater.
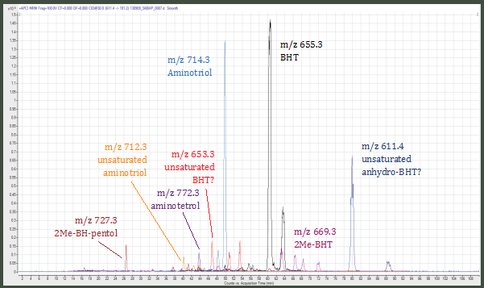
Samples are extracted with a modified Bligh & Dyer protocol and the total lipid extract is split into polarity fractions. BHPs are purified using a newly developed reverse phase UHPLC method allowing for the separation of previously co-eluting BHPs. This reverse phase method has been established using multiple reaction monitoring (MRM) with an Agilent 1290 UHPLC system coupled to a 6410 QQQ MS. BHPs are separated using three Phenomenex Kinetex C18 columns (4.6 × 150mm; 2.6μm) in series maintained at 10°C. Elution is achieved with 54:4:42 (v:v:v) MeOH: H2O: IPA isocratic mobile phase at 300μl/min. BHPs elute over a time window of ~60 minutes, allowing for resolution of identical-mass isomers (Figure 1). BHPs are collected using a fraction collector operated in time-based mode. Fraction purity is checked after fraction collection using flow injection analysis. To allow for a final purity >98% as needed for compound-specific isotope analysis, samples will be subjected to a second step, normal phase UHPLC separation. This method is currently being developed. Identification and quantification are obtained by HPLC-Q-TOF as well as UHPLC-QQQ. Isotope concentrations will be obtained by gas ion source AMS (Δ14C) and GC-IRMS (δ13C and δD of cleaved BHPs).
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Ann Pearson
Co-Investigator
Lindsay Hays
Collaborator
Stephanie Kusch
Collaborator
Elise Wilkes
Collaborator
-
RELATED OBJECTIVES:
Objective 3.2
Origins and evolution of functional biomolecules
Objective 5.1
Environment-dependent, molecular evolution in microorganisms
Objective 5.3
Biochemical adaptation to extreme environments
Objective 6.1
Effects of environmental changes on microbial ecosystems