2013 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
Task 3.4.2: Development of Direct Sampling Methodology for Analysis of Complex Organic Mixtures on Titan’s Surface
Project Summary
Photochemistry in Titan’s dense atmosphere generates a complex mixture of organic molecules that have been deposited on Titan’s surface over time. Requiring no sample pretreatment or handling, the technique of direct analysis in real time (DART), combined with an ion trap mass spectrometer having 2 dimensional mass spectroscpy capability, is shown to be an enabling experimental methodology to vaporize, ionize, and structurally characterize organic components of this mixture.
Project Progress
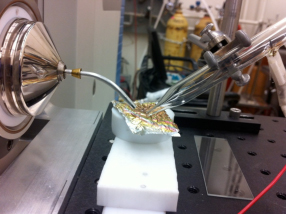
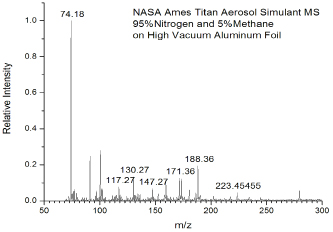
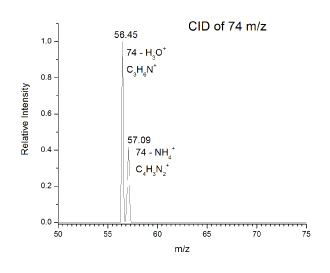
Titan aerosol simulants used in this experiment were produced using an experimental protocol developed by Dr. Ella Scimma-O’Brien at NASA Ames, employing the NASA Ames COSmIC system.1 This source comprises a pulsed discharge nozzle (PDN) composed of a copper body with a small slit on the front and two knife edge stainless steel electrodes placed along the copper slit. The electrodes are separated from the copper body by a slotted alumina plate acting as an insulator. A high voltage of -800V to -1000V DC is applied to the electrodes to produce a plasma in the jet expansion. Three synchronized solenoid valves first pulse gas into the chamber inside the copper body. The gas is then released in a pulse when two of the solenoid valves retract a poppet, allowing the mixture to escape into the COSmIC chamber through the slit.1 The pulsed plasma in the slit undergoes expansion in a supersonic jet. This causes the gas to cool down to Titan temperatures without the need for a cooling system. The aerosols are produced in the alumina cavity between the copper body and the electrodes.2 A 95% nitrogen and 5% methane gas mixture was used for the initial experiment and the aerosol was deposited on a piece of clean high vacuum aluminum foil for 38 hours. After deposition the foil was collected under nitrogen and placed in the freezer for analysis using the technique of direct analysis in real time (DART)3 with a system developed at Caltech (Caltech mini-DART). The Caltech mini-DART system comprises a quartz tube in which a discharge in helium produces helium metastables, forming a cold plasma that acts to desorb and chemically ionize the target sample under ambient conditions (laboratory air). The mini-DART was used in the angled configuration shown in Figure 1. Ions are sampled using a Thermo Scientific LTQ XL ion trap mass spectrometer. The mass spectrum of organics deposited on the foil is shown in Figure 2, revealing a complex mixture. Ions are analyzed by isolation and collisional activation in the ion trap (MS/MS). MS/MS of m/z 74 shown in Figure 3 reveals a mixture of protonated propanenitrile (m/z 56) and aminoacetonitrile (m/z 57), complexed with water and ammonia, respectively. Both of these adducts are commonly observed in DART3. The survival of these two volatile species is likely due to the samples being frozen, trapping them in the deposition layers. The loss of these species over time after thawing supports this conclusion. The production of aminoacetonitrile is of particular interest since we have shown in our earlier NAI supported studies that it undergoes tribochemical reactions in contact with water ice in Titan’s dunes to produce glycine, the simplest amino acid. Further characterization of the organics produced by COSmiC1 is underway, to be compared with other methods used to prepare Titan simulants (tholins) in the past. We anticipate that the DART methodology can be effectively employed in future missions to Titan’s surface and other space environments where complex organics are present.
1. Ricketts, C.L., et al., The coupling of a reflectron time-of-flight mass spectrometer with a cosmic simulation chamber: A powerful new tool for laboratory astrophysics. International Journal of Mass Spectrometry, 2011. 300(1): p. 26-30.
2. Broks, B.H.P., et al., Numerical investigation of the discharge characteristics of the pulsed discharge nozzle. Physical Review E, 2005. 71(3): p. 036409.
3. Cody, R.B., J.A. Laramée, and H.D. Durst, Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions. Analytical Chemistry, 2005. 77(8): p. 2297-2302.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Jesse Beauchamp
Project Investigator
Kathleen Upton
Co-Investigator
Ella Sciamma-O’Brien
Collaborator
-
RELATED OBJECTIVES: