2013 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
Task 3.4.1: Nuclear Magnetic Resonance Spectroscopy Studies of Titan Organic Analogues: Analytical Potential
Project Summary
Nuclear magnetic resonance spectroscopy (NMR) has tremendous potential for the quantitative identification of solar system organic molecules of simple to complex nature with absolute structural identification. We have investigated its potential for the elucidation of very complex mixtures of Titan aerosol haze analogues (Tholins) with identification of the major components of modest complexity using 1, 2 and 3 dimensional spectral techniques. We have also performed studies of the utility of low resolution NMR on low temperature liquid hydrocarbon mixtures analogous to Titan lake liquids towards the development of multidimensional NMR instrumentation capable of future flight missions to solar system bodies of organic composition.
Project Progress
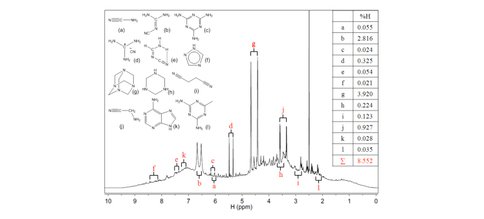
In our ongoing quest to determine analytic methodology for the quantitative determination of Titan tholin composition, with absolute molecular (structural) identification, we synthesized laboratory tholins with complete 1H, 13C and 15N isotopic composition. Not altering the chemistry of synthesis, this technique produces a tholin in which every atom of every molecule possesses a nuclear spin of I=1/2 and thus is spin coupled to every neighboring atom within the given molecule. In this way, it is possible to use nuclear magnetic resonance spectroscopy (NMR) to observe in a simple way each and every atom in a given tholin component molecule, its spin interaction with its neighboring atoms , in principle, through multidimensional spin-spin coupling techniques, determine the absolute bonding configuration of each and every atom in each molecular component in the tholin mixture as a whole. We have now completed the one dimensional (direct spin) spectrum of solution phase tholin mixtures in d6-dimethyl sulfoxide solutions in proton, carbon and nitrogen yielding the gross spectra of the tholin mixtures. Using spin decoupling we have determined the bonding relationships of the major spin signals. Using 2 dimensional spectroscopy, we have confirmed the chemical environment, bond relationship and functional group identification of the major signals and through three dimensional NMR confirmed the larger functionality, including all three of the H, C and N bonding partners. In this manner we have now, for the first time ever, with quantitative determination, identified the major small molecules in a tholin sample with complete molecular identification with the major constituents shown in Figure 1. In addition, we have gained additional functional group information without complete molecule structure regarding the less resolved structure within the spectrum, a gross mixture of very large, structurally similar large molecules tending towards polymers. In this way, we have the largest set of chemical information regarding tholin composition existing, which can predict tholin behavior as regards chemical properties, stability and reactivity. Using NMR, we have also demonstrated the thermal lability of tholins in a quantitative way by following the molecular signatures as tholins are heated as solids and as liquid solutions, verifying the importance of low temperature anaerobic approaches to tholin chemical analysis and identification for future studies if nascent properties are desired.
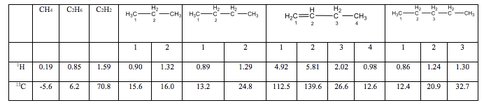
In our developing studies of the critical value of NMR to molecular quantitative and qualitative analysis of gross, un-separated mixtures of complex molecules, we performed an nmr study of the dissolved gasses expected to be present within the Titan lakes, determining the precise chemical shifts, spin coupling values and spin relaxation times for 1H and 13C nuclei in the C1-C4 hydrocarbons in a variety of pure hydrocarbon solvents at temperatures down to 190 K. The values for the most critical components in the common solvent d6-cyclohexane at 300 K are shown in Table 1. Insignificant variations in chemical shifts are seen when going to low temperature pentane solvent, though variations, which are predictable, of T1 relaxation times are observed which need to be considered when making quantitative signal comparisons or in multidimensional spectroscopy.
Publications
-
He, C., & Smith, M. A. (2013). Identification of nitrogenous organic species in Titan aerosols analogs: Nitrogen fixation routes in early atmospheres. Icarus, 226(1), 33–40. doi:10.1016/j.icarus.2013.05.013
-
He, C., Lin, G., & Smith, M. A. (2012). NMR identification of hexamethylenetetramine and its precursor in Titan tholins: Implications for Titan prebiotic chemistry. Icarus, 220(2), 627–634. doi:10.1016/j.icarus.2012.06.007
-
He, C., Lin, G., Upton, K. T., Imanaka, H., & Smith, M. A. (2012). Structural Investigation of HCN Polymer Isotopomers by Solution-State Multidimensional NMR. The Journal of Physical Chemistry A, 116(19), 4751–4759. doi:10.1021/jp301604f
-
He, C., Lin, G., Upton, K. T., Imanaka, H., & Smith, M. A. (2012). Structural Investigation of Titan Tholins by Solution-State 1 H, 13 C, and 15 N NMR: One-Dimensional and Decoupling Experiments. The Journal of Physical Chemistry A, 116(19), 4760–4767. doi:10.1021/jp3016062
-
Hörst, S. M., Yelle, R. V., Buch, A., Carrasco, N., Cernogora, G., Dutuit, O., … Vuitton, V. (2012). Formation of Amino Acids and Nucleotide Bases in a Titan Atmosphere Simulation Experiment. Astrobiology, 12(9), 809–817. doi:10.1089/ast.2011.0623
-
Somogyi, Á., Smith, M. A., Vuitton, V., Thissen, R., & Komáromi, I. (2012). Chemical ionization in the atmosphere? A model study on negatively charged “exotic” ions generated from Titan’s tholins by ultrahigh resolution MS and MS/MS. International Journal of Mass Spectrometry, 316-318, 157–163. doi:10.1016/j.ijms.2012.02.026
- Vuitton, V., Balisconi, N., Dutuit, O. & Smith, M.A. (2013). The chemistry of Titan’s atmospher. In: Meuller-Wodarg, I., Griffith, C., Lellouch, E. & Cravens, T. (Eds.). Titan: Surface, Atmosphere and Magnetosphere.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Mark Smith
Project Investigator
Chao He
Co-Investigator
Sarah Horst
Collaborator
Hiroshi Imanaka
Collaborator
Guanxin Lin
Collaborator
Arpad Somogyi
Collaborator
Kathleen Upton
Collaborator
Véronique Vuitton
Collaborator
-
RELATED OBJECTIVES:
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules