2013 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
Task 3.3.2: Trapping of Methane and Ethane in Titan Surface Materials
Project Summary
We demonstrate that solid benzene can trap significant amounts of ethane and methane within its crystal structure at Titan surface temperatures. Experiments also suggest that liquid ethane can diffuse into solid benzene, resulting in the formation of a co-crystalline structure. This implies that lake edges and evaporite basins on Titan may hold important quantities of ethane. These results can help explain the release of methane observed at the Huygens landing site, and point toward a large possible reservoir of methane and ethane hidden within Titan’s surface organics.
Project Progress
Titan is the only body in the Solar System aside from Earth that has standing liquid on its surface. Due to the low surface temperatures (90-95 K), this liquid phase is comprised of hydrocarbons, mostly methane and ethane. Active photochemistry in the atmosphere due to solar radiation and energy from Saturn’s magnetosphere generates a plethora of organic molecules, from simple molecules to compounds >10,000 Da. These species are ultimately deposited onto the surface, including into the lakes. Evaporation or other processes could potentially induce precipitation of these organics, forming ‘bathtub rings’ similar to those observed by Cassini around some of the Northern lakes. These lake evaporites may play an important role in the surface chemistry of Titan.
We focus on benzene, which has been detected by Cassini in the Titan atmosphere and was tentatively identified on the surface by Huygens. Recent work in our laboratory suggests that benzene has a very low solubility in liquid ethane (<20 mg/L), meaning it is a likely constituent of possible evaporites of Titan lakes. We investigate here whether trapping of hydrocarbons can occur under conditions representative of the Titan surface.
Ethane was condensed at Titan surface temperature (90 K) under N2 atmosphere in a custom-built cryostat. Benzene was either added to saturation or frozen separately to produce a greater quantity of crystalline material. An aliquot of the sample was transferred to a Linkam LTS 350 liquid nitrogen-cooled cryostage maintained at 90 K and ~1 bar. The sample was then analyzed within the cryostage using a Horiba Jobin Yvon LabRam HR confocal Raman microscope.
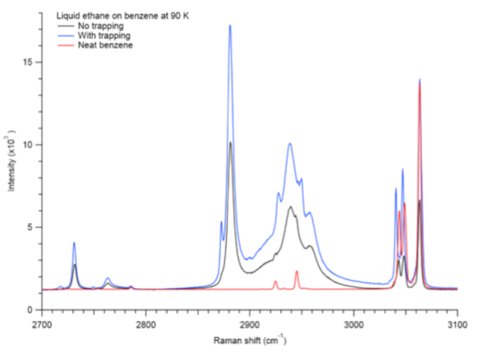
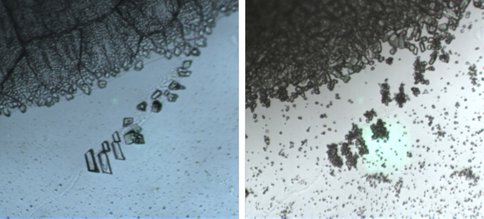
Trapping of ethane in benzene crystals was confirmed by Raman spectral shifts of both ethane and benzene peaks (Fig. 1), which shift by 10 cm-1 and 2-3 cm-1, respectively. Microscope images taken before and after (Fig. 2) suggest that benzene and ethane form a new crystal structure upon trapping.
Kinetics experiments indicate that ethane trapping reaches saturation in less than 12 hours at 90 K and ~1 bar. This suggests that trapping of ethane in a benzene evaporite would occur readily in Titan ambient conditions. Evaporite basins containing benzene may therefore act as important hydrocarbon reservoirs on Titan. Future work will involve investigation of crystalline benzene with liquid methane in a similar set of experiments.
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Robert Hodyss
Project Investigator
Patricia Beauchamp
Co-Investigator
Morgan Cable
Co-Investigator
Mathieu Choukroun
Co-Investigator
Tuan Vu
Co-Investigator
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules