2013 Annual Science Report
 NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
NASA Jet Propulsion Laboratory - Titan
Reporting | SEP 2012 – AUG 2013
Task 3.3.1: Solubility of Organics in Simulated Titan Lake Solutions
Project Summary
Widespread lakes of liquid methane and ethane were discovered on Titan by the Cassini mission in 2006, which naturally motivates questions about the solubility of surface materials in the liquid. Our goal is to measure the solubilities of Titan surface and atmospheric species in cryogenic liquid hydrocarbons, in order to constrain the composition of the hydrocarbon lakes, and provide an understanding into the nature of erosion and sedimentation on Titan. To date, we have measured the solubilities of argon and krypton in liquid methane and ethane, and the solubilities of benzene, naphthalene, and biphenyl in liquid ethane. Relatively high organic solubilities suggest that liquid hydrocarbon based weathering and sorting of surface organics should be occurring on Titan.
Project Progress
We have used ultraviolet spectroscopy of liquid hydrocarbon solutions to determine the solubilities of benzene, naphthalene, and biphenyl in liquid ethane. In a typical experiment, the analyte is added to the liquid hydrocarbon in a thermostated vessel, and ultraviolet spectra are periodically obtained. The concentration of the analyte in solution is obtained from the spectra after calibration.
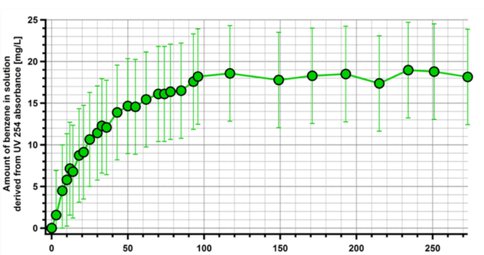
The time course of benzene dissolution in ethane at 94 K for a typical run is shown in Figure 1: this particular run had an estimated benzene surface area of 9.6 cm2 based on the quantity added and the grain size. Experimental runs with varying amounts of benzene produced different curves, but all came to the same equilibrium concentration within experimental error. When all the datapoints passed the timepoint where the concentration level became flatted were summed, the csat for benzene in ethane at 94 K was 2.37 × 10-4 mmol cm-3 (± 2.4 × 10-5 mmol cm-3) or 18.5 mg L-1 (± 2 mg L-1). Similar experiments were performed on naphthalene and biphenyl. The equilibrium saturation value for naphthalene in ethane at 94 K was 1.23 × 10-6 mmol cm-3 (± 2.6 10-8 mmol cm-3) or 0.159 mg L-1 (± 0.003 mg L-1), and for biphenyl csat in ethane at 94 K was 2.55 × 10-8 mmol cm-3 (±4.9 × 10-8 mmol cm-3) or 0.039 mg L-1 (± 0.006 mg L-1).
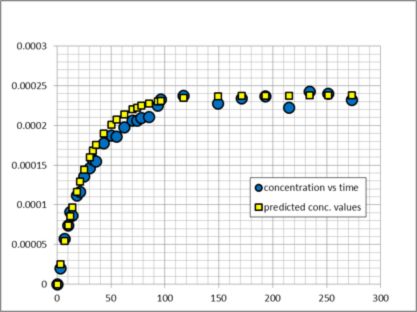
Rates of dissolution can also be obtained from our data. Figure 2 shows predicted and observed concentration values for benzene in ethane using an effective rate of keff = 3.72 × 10-6 mmol cm-2 sec-1. Rates for naphthalene and biphenyl were also obtained.
The volatile hydrocarbon cycle on Titan provides the possibilities of deposited surface organics to be dissolved or leached from surface deposits and transferred as solutions into lakes or evaporite playas. The existence of semi-permanent bodies of liquids on Titan’s surface also allows the possibility of organic compounds to be dissolved in the fluids much as salts are dissolved in terrestrial oceans and lakes. As on Earth, endorheic basins with a large collection area, or those that tap into particularly rich deposits or soluble organic molecules, may reach saturation quickly.
Our results suggest several implications for the chemistry of Titan’s lakes and surface. Reasonable assumptions of lake depth, age and the atmospheric production rate of benzene suggest that Titan’s lake should be saturated in benzene, and a sludge of excess benzene to accumulate at the bottom of any long-lived Titan lakes. It also appears likely and possible that dissolution geology, e.g, the formation of karst, is a likely process on the organic landscape of Titan. Our laboratory measured values have important implications for Titan dissolution geology as well as lake composition and limnology. They also provide constraints on the transport and movement of organic materials on Titan’s surface.
Publications
-
Hodyss, R., Choukroun, M., Sotin, C., & Beauchamp, P. (2013). The solubility of 40 Ar and 84 Kr in liquid hydrocarbons: Implications for Titan’s geological evolution. Geophysical Research Letters, 40(12), 2935–2940. doi:10.1002/grl.50630
-
PROJECT INVESTIGATORS:
-
PROJECT MEMBERS:
Robert Hodyss
Project Investigator
Michael Malaska
Co-Investigator
Patricia Beauchamp
Collaborator
Christophe Sotin
Collaborator
-
RELATED OBJECTIVES:
Objective 1.1
Formation and evolution of habitable planets.
Objective 2.2
Outer Solar System exploration
Objective 3.1
Sources of prebiotic materials and catalysts
Objective 3.2
Origins and evolution of functional biomolecules